Molecular and Morphological Characterization of Exserohilum turcicum (Passerini) Leonard and Suggs Causing Northern Corn Leaf Blight of Maize in Bihar
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Isolation of Pathogens under Standard Cultivation Conditions
2.2. Isolation and Identification of Exserohilum Turcicum
2.3. Cultural and Morphological Characterization
2.4. Molecular Characterization
2.4.1. DNA Isolation
2.4.2. PCR Amplification
3. Results
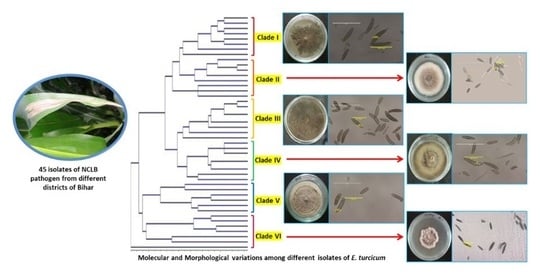
3.1. Cultural and Morphological Characterization
3.2. Molecular Characterization
3.3. Sequence Analysis and Nucleotide Data Submission in GenBank
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Passerini, G. Cladosporium paeoniae. In Just’s Botanische Jahresberichte; Gebruder Borntraeger: Leipzig, Germany, 1876; Volume 4, p. 235. [Google Scholar]
- Butler, E.J. Some diseases of cereals caused by Sclerospora graminicola. In Botanical Survey; Department of Agriculture: New Delhi, India, 1907; Volume 2, pp. 1–24. [Google Scholar]
- Blandino, M.; Galeazzi, M.; Savoia, W.; Reyneri, A. Timing of azoxystrobin + propiconazole application on maize to control northern corn leaf blight and maximize grain yield. Field Crop. Res. 2012, 139, 20–29. [Google Scholar] [CrossRef]
- Rajeshwar, R.P.; Narayan, R.; Ranga, P.; Sokka, S.R. Cultural and morphological variability among Exserohilum turcicum isolates. Int. J. Sci. Res. Publ. 2014, 3, 54–59. [Google Scholar]
- Singh, N.P. Maize in India: Production systems, constraints, and research priorities. Indian Res. J. Ext. Educ. 2005, 42, 821–833. [Google Scholar]
- Raymundo, A.D.; Hooker, A.L. Measuring the relationship between northern corn leaf blight and yield losses. Plant Dis. 1981, 65, 325–327. [Google Scholar] [CrossRef]
- Tefferi, A.; Bartholmai, B.J.; Witzig, T.E.; Li, C.Y.; Hanson, C.A.; Phyliky, R.L. Heterogeneity and clinical relevance of the intensity of CD20 and immunoglobulin light-chain expression in B-cell chronic lymphocytic leukemia. Am. J. Clin. Pathol. 1996, 106, 457–461. [Google Scholar] [CrossRef]
- Bigirwa, G.; Julian, A.M.; Adiala, E. Characterization of ugandian races of Exserohilum turcicum from Maize. Afr. Crop Sci. J. 1993, 1, 69–72. [Google Scholar]
- Mathur, K.; Thakur, R.P.; Rao, V.P.; Jadone, K.; Rathore, S.; Velazhahan, R. Pathogenic variability in Exserohilum turcicum and resistance to leaf blight in sorghum. Indian Phytopathol. 2011, 64, 32–36. [Google Scholar]
- Boora, K.S.; Frederiksen, R.A.; Magill, C.W. A molecular marker that segregates with sorghum leaf blight resistance in one cross is maternally inherited in another. Mol. Genet. Genom. 2003, 261, 317–322. [Google Scholar] [CrossRef]
- Abadi, R.; Perl-Treves, R.; Levy, Y. Molecular variability among Exserohilum turcicum isolates using RAPD (random amplified polymorphic DNA). Can. J. Plant Pathol. 1996, 18, 29–34. [Google Scholar] [CrossRef]
- Mathur, K.; Thakur, R.P.; Reddy, B.V.S. Leaf blight. In Screening Techniques for Sorghum Diseases; Information Bulletin No. 76.; Thakur, R.P., Reddy, B.V.S., Mathur, K., Eds.; International Crops Research Institute for the Semi-Arid Tropics: Patancheru, India, 2007; p. 92. [Google Scholar]
- Jones, M.J.; Dunkle, L.D. Analysis of Cochliobolus carbonum races by PCR amplification with arbitrary and gene-specific primers. Phytopathology 1993, 83, 366–370. [Google Scholar] [CrossRef]
- Khan, M.R.; Anwer, M.A. DNA and some laboratory tests of nematode suppressing efficient soil isolates of Aspergillus niger. Indian Phytopathol. 2008, 61, 212–225. [Google Scholar]
- Khan, M.R.; Anwer, M.A. Molecular and biochemical characterization of soil isolates of Aspergillus niger aggregate and an assessment of their antagonism against Rhizoctonia solani. Phytopathol. Mediterr. 2008, 46, 304–315. [Google Scholar]
- Navarro, B.L.; Ramos Romero, L.; Kistner, M.B. Assessment of physiological races of Exserohilum turcicum isolates from maize in Argentina and Brazil. Trop. Plant Pathol. 2021, 46, 371–380. [Google Scholar] [CrossRef]
- Manamgoda, D.S.; Cai, L.; McKenzie, E.H.C. A phylogenetic and taxonomic re-evaluation of the Bipolaris, Cochliobolus, Curvularia complex. Fungal Divers. 2012, 56, 131–144. [Google Scholar] [CrossRef]
- Ferdinandez, H.S.; Manamgoda, D.S.; Udayanga, D.; Deshappriya, N.; Munasinghe, M.L.A.M.S. Morphological and molecular characterization of two graminicolous Exserohilum species associated with cultivated rice and early barnyard grass from Sri Lanka. Ceylon J. Sci. 2020, 49, 381–387. [Google Scholar] [CrossRef]
- Tomioka, K.; Asami, H.; Chiba, M. Leaf spot of barnyardgrass caused by Exserohilum oryzicola in Japan and the fungal influence on rice, barley, bread wheat, durum wheat, and soybean. J. Gen. Plant Pathol. 2021, 87, 281–286. [Google Scholar] [CrossRef]
- Rasband, W.S. ImageJ OS Version U.S.; version ImageJ bundled with 64-bit Java 1.8.0_172; National Institutes of Health: Bethesda, MD, USA, 2018. Available online: https://imagej.nih.gov/ij/ (accessed on 12 April 2022).
- Saghai-Maroof, M.A.; Soliman, K.M.; Jordensen, R.A.; Allard, R.W. Ribosomal DNA spacer-length polymorphism in barley: Mendelian inheritance, chromosomal location, and population dynamics. Proc. Natl. Acad. Sci. USA 1984, 81, 8014–8018. [Google Scholar] [CrossRef]
- Dai, Y.; Gan, L.; Lan, C.; Lu, X.; Yang, X.; Gao, Z. Genetic Differentiation and Mixed Reproductive Strategies in the Northern Corn Leaf Blight Pathogen Setosphaeria turcica from Sweet Corn in Fujian Province, China. Front. Microbiol. 2021, 12, 632575. [Google Scholar] [CrossRef]
- Singh, P.; Huang, S.Y.; Hernandez, A.G.; Adhikari, P.; Tiffany, M.J.; Mideros, S.X. Genomic regions associated with virulence in Setosphaeria turcica identified by linkage mapping in a biparental population. Fungal Genet. Biol. 2022, 159, 103655. [Google Scholar] [CrossRef]
- Kroon, L.P.; Bakker, F.T.; Van den Bosch, G.B.; Bonants, P.J.; Flier, W.G. Phylogenetic analysis of Phytophthora species based on mitochondrial and nuclear DNA sequences. Fungal Genet. Biol. 2004, 41, 766–782. [Google Scholar] [CrossRef]
- Snager, F.; Coulson, A.R. A rapid method for determining sequences in DNA by primed synthesis with DNA polymerase. J. Mol. Biol. 1975, 94, 441–448. [Google Scholar] [CrossRef]
- Sievers, F.; Higgins, D.G.; Russell, D.J. (Eds.) Multiple Sequence Alignment Methods. In Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2014; Volume 1079, pp. 105–116. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schaffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Gowda, K.T.P.; Mallikarjuna, N.; Kumar, G.B.S.; Manjunath, B.; Kumar, B.R. Cultural and morphological variation in the isolates of Exserohilum turcicum the incitant of turcicum leaf blight in maize. Environ. Ecol. 2010, 28, 1826–1830. [Google Scholar]
- Sivanesan, A. New species of Exserohilum. Trans. Br. Mycol. Soc. 1986, 83, 319–329. [Google Scholar] [CrossRef]
- Misra, A.P.; Mishra, P. Variations in four different isolates of Helminthosporiurn turcicum from Sorghum vulgare. Indian Phytopathol. 1971, 24, 514–521. [Google Scholar]
- Bergquist, R.R.; Masias, O.R. Physiologic specialization in Trichometasphaeria turcica f. sp. zeae and T. turcica f. sp. sorghi in Hawaii. Phytopathology 1974, 64, 645–649. [Google Scholar] [CrossRef]
- Abebe, D.; Singburaudom, N. Morphological, Cultural and Pathogenicity variation of Exserohilum turcicum (Pass.) Leonard and Suggs. Isolates in Maize (Zea Mays L.). Kasetsart J. (Nat. Sci.) 2006, 40, 341–352. [Google Scholar]
- Varma, P.K.; Hegde, Y.R.; Kulkarni, S.; Kalappanavar, I.K. Variability in Helminthosporium sativum with respect to morphology and symptomatology. Ann. Biol. 2005, 21, 209–212. [Google Scholar]
- Bunker, R.N.; Mathur, K. Pathogenic and morphological variability of Exserohilum turcicum isolates causing leaf blight in sorghum (Sorghum bicolor). Indian J. Agric. Sci. 2010, 80, 888–892. [Google Scholar]
- CMI. Trichometaspaeria turcica. In Descriptions of Pathogenic Fungi and Bacteria; Centre for Agriculture and Bioscience International (CABI): Wallingford, UK, 1971; p. 304. [Google Scholar]
- Kimati, H.; Bach, E.E. Morphological and pathogenic comparisons of Exserohilum turcicum isolated from maize, sorghum and jhonsons grass. Summa Phytopathol. 1995, 21, 134–139. [Google Scholar]
- Merle, T.J.; Robert, L.A. Reaction of inbred lines of corn to Helminthosporium turcicum pass in different seasons. Agron. J. 1957, 49, 481–483. [Google Scholar]
- Jenkins, M.T.; Robert, A.L.; Findely, W.R. Genetic studies of resistance to Helminthosporium turcicum. Agron. J. 1957, 49, 197–201. [Google Scholar] [CrossRef]
- Adiopla, E.; Lipps, P.E.; Madden, L.V. Reaction of maize cultivars from Uganda to Exserhilum turcicum. Phytopathology 1993, 83, 217–223. [Google Scholar] [CrossRef]
- Kutawa, A.B.; Sijam, K.; Ahmad, K.; Seman, Z.A.; Razak, M.S.F.A.; Abdullah, N. Characterisation and pathological variability of Exserohilum turcicum responsible for causing northern corn leaf blight (NCLB) disease in Malaysia. Malays. J. Microbiol. 2017, 13, 41–49. [Google Scholar]
- Kumar, S.; Verma, S. Variability in Plant Pathogens and Tools for its Characterization. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 2887–2902. [Google Scholar] [CrossRef]
- Rajeshwar, R.T.; Reddy, P.N.; Reddy, R.R. Pathogenic Variability of Isolates of Exserohilum turcicum, Incitant of Leaf Blight of Maize. Indian J. Plant Prot. 2013, 41, 72–75. [Google Scholar]
- Robert, A.L.; Sprague, G.F. Adaption of the corn leaf blight fungus to a resistant and susceptible corn host. Phytopathology 1960, 50, 261–263. [Google Scholar]
- Sisterna, M.N. Study on the pathogenicity of Exserohilum turcicum in Argentina. Rev. Fac. Agron. Univ. Nac. Plata 1985, 61/62, 169–174. [Google Scholar]
- Adipala, E. Reactions of maize genotypes to Exserohilum turcicum in different agro-climatic zones of Uganda. East Afr. Agric. For. J. 1994, 59, 213–218. [Google Scholar] [CrossRef]
- Taylor, J.W.; Jacobson, D.J.; Fisher, M.C. The evolution of asexual fungi: Reproduction, speciation and classification. Annu. Rev. Phytopathol. 1999, 37, 197–246. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.W.; Branco, S.; Gao, C.; Hann-Soden, C.; Montoya, L.; Sylvain, I.; Gladieux, P. Sources of fungal genetic variation and associating it with phenotypic diversity. Microbiol. Spectr. 2017, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- McDonald, B.A.; Linde, C. Pathogen population genetics, evolutionary potential, and durable resistance. Annu. Rev. Phytopathol. 2002, 40, 349–379. [Google Scholar] [CrossRef] [PubMed]
- Borchardt, D.S.; Welz, H.G.; Geiger, H.H. Genetic structure of Setosphaeria turcica populations in tropical and temperate climates. Phytopathology 1998, 88, 322–329. [Google Scholar] [CrossRef]
- Zeigler, R.S.; Scott, R.P.; Leung, H. Evidence of parasexual exchange of DNA in the rice blast fungus challenges its exclusive clonality. Phytopathology 1997, 87, 284–294. [Google Scholar] [CrossRef]
- Begoude, B.A.; Slippers, B.; Wingfield, M.J.; Roux, J. Botryosphaeriaceae associated with Terminalia catappa in Cameroon, South Africa and Madagascar. Mycol. Prog. 2010, 9, 101–123. [Google Scholar] [CrossRef]
- Mohali, S.; Slippers, B.; Wingfield, M.J. Two new Fusicoccum species from Acacia and Eucalyptus in Venezuela based on morphology and DNA sequence data. J. Mycol. Res. 2010, 110, 405–413. [Google Scholar] [CrossRef]
- Burdon, J.J.; Silk, J. Sources and patterns of diversity in plant pathogenic fungi. Phytopathology 1997, 87, 664–669. [Google Scholar] [CrossRef]
- Zeigler, R.S.; Cuoc, L.X.; Scott, R.P.; Bernado, M.A.; Chen, D.H.; Valent, B.; Nelson, R.J. The relationship between lineage and virulence in Pyricularia grisea in the Philippines. Phytopathology 1995, 85, 443–451. [Google Scholar] [CrossRef]
- Bunkoed, W.; Kasam, S.; Chaijuckam, P.; Yhamsoongnern, J.; Prathuangwong, S. Sexual reproduction of Setosphaeria turcica in natural corn fields in Thailand. Agric. Nat. Resour. 2014, 48, 175–182. [Google Scholar]









| Isolates | Colony | Conidia | Sporulation /Microscopic Field | Conidia/mL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type | Color | Radial Growth (mm) | Margin | Pigmentation | Margin Color | Color | No. of Septa | Length (µm) | Width (µm) | Shape | |||
| BhEt1 | Flattened | Blackish brown | 90.0 | Regular | Brownish | Brown | Brown | 3–6 | 101.76 | 20 | Elongated, ellipsoidal, slightly curved fusiform with protruding hilum | 12 | 9.75 × 105 |
| BhEt2 | Flattened | Olivaceous brown | 88.1 | Regular | Brownish | Brown | Dark Brown | 3–9 | 72.35 | 15.29 | Slightly curved | 18 | 9.22 × 105 |
| BhEt3 | Fluffy Raised cottony | Whitish black | 67.3 | Regular | Grayish Black | Dark Brown | 3–8 | 55.88 | 11.18 | Elongated, ellipsoidal, obclavate to fusiform, spindle with protruding hilum | 22 | 5.45 × 105 | |
| BhEt4 | Flattened | Olivaceous brown | 85.4 | Regular | Brownish | Brown | Brown | 3–7 | 71.76 | 17.06 | Slightly curved | 9 | 8.12 × 105 |
| BhEt5 | Raised Cottony | Whitish black | 70.3 | Regular | Whitish Black | White | Olivaceous brown | 3–10 | 120 | 13.53 | Slightly curved | 15 | 5.55 × 105 |
| BhEt6 | Raised Cottony | Whitish black | 75.6 | Regular | Whitish Black | White | Brown | 3–7 | 137.65 | 16.47 | Slightly curved | 12 | 6.8 × 105 |
| BhEt7 | Raised Cottony | Whitish black | 85.8 | Regular | Whitish Black | White | Dark Brown | 3–7 | 121.18 | 15.29 | Obclavate | 8 | 8.65 × 105 |
| BhEt8 | Raised Cottony | Whitish black | 77.5 | Regular | Whitish Black | White | Dark Brown | 3–8 | 117.65 | 21.18 | Obclavate | 16 | 6.82 × 105 |
| BhEt9 | Fluffy Raised cottony | Grayish | 79.6 | Irregular | Grayish Black | Gray | Dark Brown | 2–5 | 71.18 | 10 | Obclavate | 10 | 7.98 × 105 |
| BeEt1 | Slightly Raised Fluffy | Whitish black | 85.8 | Irregular | Whitish Black | Black | Olivaceous brown | 3–7 | 88.24 | 17.65 | Slightly curved | 8 | 8.85 × 105 |
| BeEt2 | Flattened | Olivaceous brown | 88.1 | Regular | Brownish | Brown | Dark brown | 2–5 | 74.71 | 11.76 | Slightly curved | 13 | 9.65 × 105 |
| BeEt3 | Slightly Raised Fluffy | Blackish brown | 90.0 | Regular | Grayish Black | Brown | Olivaceous brown | 3–9 | 104.71 | 15.29 | Slightly curved | 10 | 9.53 × 105 |
| BeEt4 | Slightly Raised Fluffy | Olivaceous brown | 89.2 | Regular | Brownish | Brown | Dark brown | 3–8 | 102.35 | 17.06 | Obclavate | 8 | 9.44 × 105 |
| BeEt5 | Slightly Raised Fluffy | Olivaceous brown | 64.6 | Irregular | Whitish black | White | Olivaceous brown | 4–10 | 122.35 | 15.29 | Slightly curved | 10 | 3.23 × 105 |
| BeEt6 | Flattened | Grayish | 90.0 | Regular | Grayish Black | Gray | Brown | 3–8 | 105.29 | 10 | Slightly curved | 10 | 9.88 × 105 |
| BeEt7 | Flattened | Grayish | 90.0 | Regular | Grayish Black | Gray | Dark brown | 3–11 | 116.47 | 16.47 | Slightly curved | 12 | 9.55 × 105 |
| BeEt8 | Slightly Raised Fluffy | Olivaceous brown | 87.1 | Irregular | Whitish black | Gray | Olivaceous brown | 3–7 | 120.94 | 20.06 | Slightly curved | 9 | 8.95 × 105 |
| BeEt9 | Slightly Raised Fluffy | Grayish | 90.0 | Regular | Brownish | Gray | Olivaceous brown | 3–10 | 117.65 | 15.88 | Slightly curved | 8 | 9.65 × 105 |
| KhEt1 | Flattened | Grayish | 90.0 | Regular | Grayish Black | Gray | Brown | 3–12 | 120.59 | 21.76 | Slightly curved | 12 | 9.54 × 105 |
| KhEt2 | Fluffy Raised Cottony | Olivaceous brown | 90.0 | Regular | Brownish | White | Dark Brown | 2–9 | 130.59 | 24.12 | Slightly curved | 18 | 9.48 × 105 |
| KhEt3 | Flattened | Grayish | 90.0 | Regular | Grayish Black | Gray | Dark Brown | 3–9 | 112.35 | 18.24 | Slightly curved | 9 | 9.68 × 105 |
| KhEt4 | Fluffy Raised Cottony | Grayish | 86.4 | Regular | Grayish Black | Gray | Dark Brown | 3–10 | 67.06 | 12.94 | Slightly curved | 22 | 8.86 × 105 |
| KhEt5 | Slightly Raised Fluffy | Olivaceous brown | 75.5 | Irregular | Brownish | Brown | Brown | 3–12 | 64.71 | 12.35 | Slightly curved | 26 | 7.12 × 105 |
| KhEt6 | Fluffy Raised Cottony | Whitish black | 80.6 | Irregular | Whitish black | Black | Brown | 3–10 | 69.41 | 11.76 | Slightly curved | 20 | 7.85 × 105 |
| KhEt7 | Flattened | Olivaceous brown | 80.9 | Regular | Whitish black | Gray | Dark Brown | 3–7 | 112.94 | 15.29 | Slightly curved | 10 | 7.89 × 105 |
| KhEt8 | Flattened | Olivaceous brown | 90.0 | Regular | Brownish | Brown | Olivaceous brown | 3–10 | 121.76 | 20 | Slightly curved | 16 | 9.78 × 105 |
| KhEt9 | Flattened | Grayish | 90.0 | Regular | Grayish black | Gray | Brown | 2–6 | 81.18 | 25.29 | Slightly curved | 12 | 9.75 × 105 |
| KaEt1 | Slightly Raised Fluffy | Olivaceous brown | 80.8 | Regular | Brownish | White | Brown | 2–5 | 99.41 | 21.76 | Slightly curved | 8 | 8.45 × 105 |
| KaEt2 | Slightly Raised Fluffy | Whitish black | 65.5 | Irregular | Whitish black | Black | Olivaceous brown | 3–8 | 116.47 | 20.59 | Slightly curved | 10 | 3.12 × 105 |
| KaEt3 | Fluffy Raised Cottony | Olivaceous brown | 85.6 | Regular | Brownish | White | Brown | 3–7 | 102.94 | 10.59 | Slightly curved | 8 | 8.92 × 105 |
| KaEt4 | Fluffy Raised Cottony | Grayish | 90.0 | Regular | Grayish Black | Gray | Brown | 3–12 | 65.29 | 11.76 | Obclavate | 25 | 9.6 × 105 |
| KaEt5 | Fluffy Raised Cottony | Whitish black | 64.7 | Regular | Whitish black | White | Brown | 3–7 | 77.06 | 20.59 | Obclavate | 20 | 3.08 × 105 |
| KaEt6 | Fluffy Raised Cottony | Grayish | 90.0 | Regular | Grayish Black | Gray | Dark Brown | 3–10 | 59.41 | 14.12 | Obclavate | 24 | 9.72 × 105 |
| KaEt7 | Fluffy Raised Cottony | Grayish | 86.4 | Regular | Grayish Black | Gray | Olivaceous brown | 2–8 | 77.65 | 10 | Obclavate | 13 | 9.42 × 105 |
| KaEt8 | Fluffy Raised Cottony | Olivaceous brown | 90.0 | Regular | Whitish black | Gray | Olivaceous brown | 3–6 | 100 | 18.82 | Obclavate | 15 | 9.66 × 105 |
| KaEt9 | Slightly Raised Fluffy | Olivaceous brown | 86.5 | Regular | Brownish | Brown | Olivaceous brown | 2–7 | 115.29 | 17.06 | Obclavate | 12 | 8.96 × 105 |
| SaEt1 | Fluffy Raised Cottony | Grayish | 90.0 | Regular | Grayish Black | Gray | Dark Brown | 3–8 | 55.29 | 15.29 | Obclavate | 24 | 9.88 × 105 |
| SaEt2 | Fluffy Raised Cottony | Grayish | 90.0 | Regular | Grayish Black | Gray | Dark Brown | 3–12 | 87.65 | 13.53 | Obclavate | 20 | 9.84 × 105 |
| SaEt3 | Flattened | Olivaceous brown | 80.1 | Regular | Brownish | Brown | Brown | 3–12 | 59.41 | 16.47 | Slightly curved | 16 | 8.75 × 105 |
| SaEt4 | Slightly Raised Fluffy | Blackish brown | 89.9 | Regular | Grayish Black | Brown | Brown | 3–10 | 144.12 | 25.88 | Slightly curved | 13 | 9.35 × 105 |
| SaEt5 | Flattened | Blackish brown | 90.0 | Regular | Grayish Black | Brown | Dark Brown | 3–8 | 83.53 | 19.41 | Obclavate | 16 | 9.76 × 105 |
| SaEt6 | Fluffy Raised Cottony | Grayish | 90.0 | Regular | Grayish Black | Gray | Olivaceous brown | 2–9 | 102.35 | 11.18 | Slightly curved | 22 | 9.32 × 105 |
| SaEt7 | Fluffy Raised Cottony | Grayish | 90.0 | Regular | Grayish Black | Gray | Dark Brown | 3–10 | 110.59 | 18.82 | Slightly curved | 20 | 9.47 × 105 |
| SaEt8 | Flattened | Grayish | 89.8 | Regular | Grayish Black | Gray | Brown | 4–9 | 81.18 | 15.88 | Slightly curved | 26 | 9.12 × 105 |
| SaEt9 | Flattened | Olivaceous brown | 86.8 | Regular | Brownish | Brown | Brown | 3–10 | 52.94 | 11.18 | Slightly curved | 15 | 9.44 × 105 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anwer, M.A.; Niwas, R.; Ranjan, T.; Mandal, S.S.; Ansar, M.; Srivastava, J.N.; Kumar, J.; Jain, K.; Kumari, N.; Bharti, A. Molecular and Morphological Characterization of Exserohilum turcicum (Passerini) Leonard and Suggs Causing Northern Corn Leaf Blight of Maize in Bihar. Bioengineering 2022, 9, 403. https://doi.org/10.3390/bioengineering9080403
Anwer MA, Niwas R, Ranjan T, Mandal SS, Ansar M, Srivastava JN, Kumar J, Jain K, Kumari N, Bharti A. Molecular and Morphological Characterization of Exserohilum turcicum (Passerini) Leonard and Suggs Causing Northern Corn Leaf Blight of Maize in Bihar. Bioengineering. 2022; 9(8):403. https://doi.org/10.3390/bioengineering9080403
Chicago/Turabian StyleAnwer, Md Arshad, Ram Niwas, Tushar Ranjan, Shyam Sundar Mandal, Mohammad Ansar, Jitendra Nath Srivastava, Jitesh Kumar, Khushbu Jain, Neha Kumari, and Aditya Bharti. 2022. "Molecular and Morphological Characterization of Exserohilum turcicum (Passerini) Leonard and Suggs Causing Northern Corn Leaf Blight of Maize in Bihar" Bioengineering 9, no. 8: 403. https://doi.org/10.3390/bioengineering9080403