Species-Level Differences in Osmoprotectants and Antioxidants Contribute to Stress Tolerance of Quercus robur L., and Q. cerris L. Seedlings under Water Deficit and High Temperatures
Abstract
:1. Introduction
- The drought and heat stresses result in specific changes in the production of both individual and/or pattern of osmoprotectants and antioxidants.
- Species-specific constitutive levels of antioxidants, osmoprotectants, and hormones predispose the studied oak species differently to unfavorable environmental conditions.
- The magnitude of the examined biochemical responses to both stresses depends on the species-specific features and on the nature of stress.
- Functional and metabolic relationships among the various metabolites contribute to the physiological performance and stress tolerance capacity of the investigated oak species.
2. Results
2.1. Effect of Drought and Heat Stress on Osmolyte and DMSP Accumulation
2.2. Effects of Water Deficit and High Temperatures on the Antioxidant Defense System
2.3. Leaf Nitrogen and Sulfur Status in Response to Water Deficit and High Temperatures
2.4. Changes in Leaf IAA and ABA Levels Induced by Soil Drying Conditions and High Temperatures
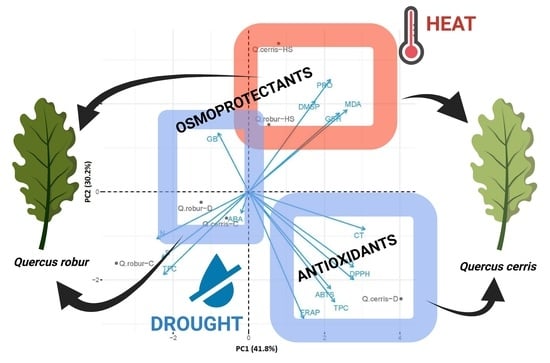
2.5. PCA Analysis
2.6. Heat Map with Bi-Cluster Analysis
2.7. Correlation Analysis
3. Discussion
3.1. Species-Specific Accumulation of Osmotically Active Substances Is Induced Differently by Drought and Elevated Temperatures
3.2. Foliar Antioxidant Defense Systems Are Activated by Drought but Less by Heat
3.3. Tolerance to Soil Water Deficit and High Temperature May Rely on Compensation Mechanisms
3.4. Drought and Heat Stress Impact the Hormone Balance Differently
4. Materials and Methods
4.1. Plant Material and Experimental Design
- (1)
- Control (C): plants were well-watered daily to maintain SWC in the range of 32–38% and were grown at ambient temperature (daytime temperature: 25–28 °C; nighttime: 10–14 °C);
- (2)
- (3)
- Heat stress (HS): plants were exposed to daily air temperatures ranging between 33–47 °C (night temperatures were about 25–30 °C), using a small enclosure chamber in the greenhouse for 6 days [64]. During the heat treatment, plants were regularly watered to maintain the soil’s maximum water capacity, and the daily air temperature was continuously monitored with thermo-sensors.
4.2. Measurements of Osmolytes’ Accumulation:
4.3. Assays of Antioxidant Defense Systems
- (1)
- Lipid peroxidation was measured as malondialdehyde (MDA) equivalent which is the secondary end-product of the oxidation of polyunsaturated fatty acids. Determination of MDA was carried out using the acid-catalyzed complexation reaction between MDA and thiobarbituric acid [110]; results are expressed as nmol MDA equivalents on a DW basis.
- (2)
- Total non-protein thiol compounds were measured according to a modified colorimetric assay based on measuring the absorbance of yellow Ellman’s reagent (5,5′-dithiobis-(2-nitrobenyoic acid; DTNB) reduced by sulfhydryl compounds at 413 nm. After construction of the calibration curve where we used reduced glutathione (GSH) as standard, total non-protein thiol compounds were expressed as GSH equivalents on a DW basis [111].
- (3)
- Trolox® Equivalent Antioxidant Capacity (TEAC) was estimated with the 2,2′-azinobis-(3-ethylbenzothiozoline-6-sulfonic acid) (ABTS) assay based on the capability of the ethanolic extract to scavenge ABTS radicals [112]. Data were expressed as TEAC on a DW basis.
- (4)
- The 2,2-di-phenyl-1-picrylhydrazyl (DPPH) radical scavenging assay was also applied to measure the antioxidant activity level of ethanolic extracts expressed as TEAC on a DW basis [113].
- (5)
- The Ferric Reducing Antioxidant Power (FRAP) assay was performed to estimate the ability of the plant extract to reduce the ferric 2, 4, 6-tripyridyl-S-triazine complex [Fe3+-(TPTZ)2]3− to the intensively blue-colored ferrous complex (Fe2+-(TPTZ)2]2− in acidic medium [114]. Data are expressed as TEAC on a DW basis.
- (6)
- Total phenolic content (TPC) was measured by the Folin–Ciocalteu method [115]. Data are expressed as mg of Gallic Acid Equivalents (GAE) on a DW basis.
- (7)
- Total flavonoid content (TFC) was measured by the aluminum chloride colorimetric method [116]. Data are expressed as mg of Quercetin Equivalent (QE) on a DW basis.
- (8)
- Condensed tannins (CT) content was determined from methanolic extracts by using the butanol-HCl-Fe (III) method [117]. Data were expressed as leucocyanidin equivalents (LE) on a DW basis.
4.4. Elemental Analysis of Inorganic Nitrogen and Sulfur
4.5. Determination of Leaf Indol-3-Acetic Acid (IAA) and Abscisic Acid (ABA)
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Edenhofer, O.; Pichs-Madruga, R.; Sokona, Y.; Minx, J.C.; Farahani, E.; Kadner, S.; Seyboth, K.; Adler, A.; Baum, I.; Brunner, S.; et al. Climate Change 2014: Mitigation of Climate Change; Cambridge University Press: Cambridge, UK, 2015. [Google Scholar]
- Madrigal-González, J.; Herrero, A.; Ruiz-Benito, P.; Zavala, M.A. Resilience to Drought in a Dry Forest: Insights from Demographic Rates. For. Ecol. Manag. 2017, 389, 167–175. [Google Scholar] [CrossRef]
- Takahashi, S.; Badger, R.M. Photoprotection in plants: A new light on photosystem II damage. Trends Plant Sci. 2011, 16, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Kebert, M.; Rapparini, F.; Neri, L.; Bertazza, G.; Orlović, S.; Biondi, S. Copper-Induced Responses in Poplar Clones Are Associated with Genotype- and Organ-Specific Changes in Peroxidase Activity and Proline, Polyamine, ABA, and IAA Levels. J. Plant Growth Regul. 2017, 36, 131–147. [Google Scholar] [CrossRef]
- Khaleghi, A.; Naderi, R.; Brunetti, C.; Maserti, B.E.; Salami, A.S.; Babalar, M. Morphological, physiochemical and antioxidant responses of Maclura pomifera to drought stress. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bhusal, N.; Han, S.G.; Yoon, T.M. Impact of drought stress on photosynthetic response, leaf water potential, and stem sap flow in two cultivars of bi-leader apple trees (Malus× domestica Borkh.). Sci. Hortic. 2019, 246, 535–543. [Google Scholar] [CrossRef]
- Pintó-Marijuan, M.; Munné-Bosch, S. Photo-oxidative stress markers as a measure of abiotic stress-induced leaf senescence: Advantages and limitations. J. Exp. Bot. 2014, 65, 3845–3857. [Google Scholar] [CrossRef] [Green Version]
- Cruz de Carvalho, M.H. Drought stress and reactive oxygen species: Production, scavenging and signaling. Plant Signal. Behav. 2007, 3, 156–165. [Google Scholar] [CrossRef] [Green Version]
- Pospíšil, P. Production of Reactive Oxygen Species by Photosystem II as a Response to Light and Temperature Stress. Front. Plant Sci. 2016, 7, 1950. [Google Scholar] [CrossRef]
- Mittler, R. ROS are good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Morales, M.; Munné-Bosch, S. Oxidative Stress: A Master Regulator of Plant Trade-Offs? Trends Plant Sci. 2016, 21, 996–999. [Google Scholar] [CrossRef]
- Kwak, J.M.; Nguyen, V.; Schroeder, J.I. The role of reactive oxygen species in hormonal responses. Plant Physiol. 2006, 141, 323–329. [Google Scholar] [CrossRef] [Green Version]
- Tausz, M.; Grill, D. The Role of Glutathione in Stress Adaptation of Plants. In Proceedings of the Plant Adaptation to Stress, Kuiper Haren, The Netherlands, 25 June 1999. [Google Scholar]
- Hasanuzzaman, M.; Nahar, K.; Anee, T.I.; Fujita, M. Glutathione in Plants: Biosynthesis and Physiological Role in Environmental Stress Tolerance. Physiol. Mol. Biol. Plants 2017, 23, 249–268. [Google Scholar] [CrossRef]
- Khanna-Chopra, R.; Semwal, V.K. Ecophysiology and Response of Plants Under High Temperature Stress. In Plant Ecophysiology and Adaptation under Climate Change: Mechanisms and Perspectives I; Springer: Singapore, 2020; pp. 295–329. [Google Scholar] [CrossRef]
- Biswal, U.C.; Raval, M.K. Significance of Glutathione to Plant Adaptation to the Environment; Springer Science & Business Media: New York, NY, USA, 2001; Volume 2. [Google Scholar] [CrossRef]
- Rennenberg, H.; Loreto, F.; Polle, A.; Brilli, F.; Fares, S.; Beniwal, R.S.; Gessler, A. Physiological Responses of Forest Trees to Heat and Drought. Plant Biol. 2006, 8, 556–571. [Google Scholar] [CrossRef]
- Ballizany, W.L.; Hofmann, R.W.; Jahufer, M.Z.Z.; Barrett, B.A. Variation for Constitutive Flavonols and Morphological Traits in a New White Clover Population. Environ. Exp. Bot. 2014, 105, 65–69. [Google Scholar] [CrossRef]
- Constabel, C.P.; Yoshida, K.; Walker, V. Diverse ecological roles of plant tannins: Plant defense and beyond. Recent Adv. Polyphen. Res. 2014, 4, 115–142. [Google Scholar]
- Top, S.M.; Preston, C.M.; Dukes, J.S.; Tharayil, N. Climate Influences the Content and Chemical Composition of Foliar Tannins in Green and Senesced Tissues of Quercus Rubra. Front. Plant Sci. 2017, 8, 423. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Sánchez, M.; Aroca, R.; Muñoz, Y.; Polón, R.; Ruiz-Lozano, J.M. The Arbuscular Mycorrhizal Symbiosis Enhances the Photosynthetic Efficiency and the Antioxidative Response of Rice Plants Subjected to Drought Stress. J. Plant Physiol. 2010, 167, 862–869. [Google Scholar] [CrossRef]
- Kishor, P.K.; Sangam, S.; Amrutha, R.N.; Laxmi, P.S.; Naidu, K.R.; Rao, K.S.; Rao, S.; Reddy, K.J.; Theriappan, P.; Sreenivasulu, N. Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: Its implications in plant growth and abiotic stress tolerance. Curr. Sci. 2005, 88, 424–438. [Google Scholar]
- Chen, T.H.; Murata, N. Glycinebetaine: An effective protectant against abiotic stress in plants. Trends Plant Sci. 2008, 13, 499–505. [Google Scholar] [CrossRef]
- Otte, M.L.; Wilson, G.; Morris, J.T.; Moran, B.M. Dimethylsulphoniopropionate (DMSP) and related compounds in higher plants. J. Exp. Bot. 2004, 55, 1919–1925. [Google Scholar] [CrossRef] [Green Version]
- Stefels, J. Physiological Aspects of the Production and Conversion of DMSP in Marine Algae and Higher Plants. J. Sea Res. 2000, 43, 183–197. [Google Scholar] [CrossRef]
- Slama, I.; Abdelly, C.; Bouchereau, A.; Flowers, T.; Savouré, A. Diversity, distribution and roles of osmoprotective compounds accumulated in halophytes under abiotic stress. Ann. Bot. 2015, 115, 433–447. [Google Scholar] [CrossRef] [Green Version]
- Sunda, W.; Kieber, D.J.; Kiene, R.P.; Huntsman, S. An Antioxidant Function for DMSP and DMS in Marine Algae. Nature 2002, 418, 317–320. [Google Scholar] [CrossRef]
- Gururani, M.A.; Mohanta, T.K.; Bae, H. Current understanding of the interplay between phytohormones and photosynthesis under environmental stress. Int. J. Mol. Sci. 2015, 16, 19055–19085. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Ci, D.; Tian, M.; Zhang, D. Comparison of the Physiological Effects and Transcriptome Responses of Populus Simonii under Different Abiotic Stresses. Plant Mol. Biol. 2014, 86, 139–156. [Google Scholar] [CrossRef]
- Zia, R.; Nawaz, M.S.; Siddique, M.J.; Hakim, S.; Imran, A. Plant Survival under Drought Stress: Implications, Adaptive Responses, and Integrated Rhizosphere Management Strategy for Stress Mitigation. Microbiol. Res. 2021, 242, 126626. [Google Scholar] [CrossRef]
- Landi, M.; Cotrozzi, L.; Pellegrini, E.; Remorini, D.; Tonelli, M.; Trivellini, A.; Nali, C.; Guidi, L.; Massai, R.; Vernieri, P.; et al. When “Thirsty” Means “Less Able to Activate the Signalling Wave Trigged by a Pulse of Ozone”: A Case of Study in Two Mediterranean Deciduous Oak Species with Different Drought Sensitivity. Sci. Total Environ. 2019, 657, 379–390. [Google Scholar] [CrossRef]
- Bhusal, N.; Lee, M.; Lee, H.; Adhikari, A.; Han, A.R.; Han, A.; Kim, H.S. Evaluation of morphological, physiological, and biochemical traits for assessing drought resistance in eleven tree species. Sci. Total Environ. 2021, 779, 146466. [Google Scholar] [CrossRef] [PubMed]
- Pintó-Marijuan, M.; Munné-Bosch, S. Ecophysiology of invasive plants: Osmotic adjustment and antioxidants. Trends Plant Sci. 2013, 18, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Kesić, L.; Stojnić, S.; Orlović, S.; Pavlović, L.; Lozjanin, R.; Tepavac, A.; Vaštag, E. Variability of the morphological characteristics of pedunculate (Quercus robur L.), sessile (Q. petraea (Matt.) Lieb.) and Turkey oak (Q. cerris L.) acorns. Topola/Poplar 2018, 201, 187–201. [Google Scholar]
- Nixon, K.C. Global and Neotropical Distribution and Diversity of Oak (Genus) and Oak Forests. Ecol. Conserv. Neotrop. Montane Oak For. 2006, 185, 3–13. [Google Scholar] [CrossRef]
- García-Plazaola, J.I.; Hernández, A.; Fernández-Marín, B.; Esteban, R.; Peguero-Pina, J.J.; Verhoeven, A.; Cavender-Bares, J. Photoprotective Mechanisms in the Genus in Response to Winter Cold and Summer Drought. In Oaks Physiological Ecology. Exploring the Functional Diversity of Genus Quercus L.; Springer: Cham, Switzerland, 2017; pp. 361–391. [Google Scholar] [CrossRef]
- Corcuera, L.; Camarero, J.J.; Gil-Pelegrín, E. Functional groups in Quercus species derived from the analysis of pressure–volume curves. Trees 2022, 16, 465–472. [Google Scholar] [CrossRef]
- Simeone, M.C.; Zhelev, P.; Kandemir, G. Technical Guidelines for Genetic Conservation and Use of Turkey Oak (Quercus cerris); EUFORGEN Secretariat: Bonn, Germany, 2019. [Google Scholar]
- EUFORGEN European Forest Genetic Resources Programme. Available online: https://www.euforgen.org/ (accessed on 6 May 2022).
- Kostić, S.; Kesić, L.; Matović, B.; Orlović, S.; Stojnić, S.; Stojanović, D.B. Soil properties are significant modifiers of pedunculate oak (Quercus robur L.) radial increment variations and their sensitivity to drought. Dendrochronologia 2021, 67, 125838. [Google Scholar] [CrossRef]
- Stojanović, D.B.; Matović, B.; Orlović, S.; Kržič, A.; Trudić, B.; Galić, Z.; Stojnić, S.; Pekeč, S. Future of the Main Important Forest Tree Species in Serbia from the Climate Change Perspective. South-East Eur. For. SEEFOR 2014, 5, 117–124. [Google Scholar] [CrossRef] [Green Version]
- Hansen, U.; Schneiderheinze, J.; Stadelmann, S.; Rank, B. The α-tocopherol content of leaves of pedunculate oak (Quercus robur L.)-variation over the growing season and along the vertical light gradient in the canopy. J. Plant Physiol. 2003, 160, 91–96. [Google Scholar] [CrossRef]
- Spieß, N.; Oufir, M.; Matušíková, I.; Stierschneider, M.; Kopecky, D.; Homolka, A.; Burg, K.; Fluch, S.; Hausman, J.F.; Wilhelm, E. Ecophysiological and transcriptomic responses of oak (Quercus robur) to long-term drought exposure and rewatering. Environ. Exp. Bot. 2012, 77, 117–126. [Google Scholar] [CrossRef]
- Hu, B.; Simon, J.; Rennenberg, H. Drought and air warming affect the species-specific levels of stress-related foliar metabolites of three oak species on acidic and calcareous soil. Tree Physiol. 2013, 33, 489–504. [Google Scholar] [CrossRef]
- Hu, B.; Simon, J.; Günthardt-Goerg, M.S.; Arend, M.; Kuster, T.M.; Rennenberg, H. Changes in the dynamics of foliar N metabolites in oak saplings by drought and air warming depend on species and soil type. PLoS ONE 2015, 10, e0126701. [Google Scholar] [CrossRef]
- Bojović, M.; Nikolić, N.; Borišev, M.; Pajević, S.; Horák, R.; Pavlović, L.; Vaštag, E. The effect of drought stress and recovery on pedunculate oak populations grown in semi-controlled conditions. Topola 2017, 200, 193–207. [Google Scholar]
- Krasensky, J.; Jonak, C. Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J. Exp. Bot. 2012, 63, 1593–1608. [Google Scholar] [CrossRef] [Green Version]
- Niinemets, Ü. Uncovering the hidden facets of drought stress: Secondary metabolites make the difference. Tree Physiol. 2016, 36, 129–132. [Google Scholar] [CrossRef]
- Rennenberg, H.; Herschbach, C. Sulfur Compounds in Multiple Compensation Reactions of Abiotic Stress Responses. Sulfur Metab. Plants 2012, 1, 203–215. [Google Scholar] [CrossRef]
- Owen, S.M.; Peñuelas, J. Opportunistic emissions of volatile isoprenoids. Trends Plant Sci. 2005, 10, 420–426. [Google Scholar] [CrossRef]
- Monaghan, P.; Metcalfe, N.B.; Torres, R. Oxidative stress as a mediator of life history trade-offs: Mechanisms, measurements and interpretation. Ecol. Lett. 2009, 12, 75–92. [Google Scholar] [CrossRef]
- Horak, R.; Župunski, M.; Pajević, S.; Borišev, M.; Arsenov, D.; Nikolić, N.; Orlović, S. Carbon assimilation in oak (Quercus spp.) populations under acute and chronic high-temperature stress. Photosynthetica 2019, 57, 875–889. [Google Scholar] [CrossRef] [Green Version]
- Bojović, M.; Nikolić, N.; Borišev, M.; Pajević, S.; Horak, R.; Orlović, S.; Lozjanin, R. The impact of drought on the physiological characteristics of half-sib lines of Turkey oak (Quercus cerris L.). Glas. Sumar. Fak. 2019, 119, 9–32. [Google Scholar] [CrossRef]
- Bowditch, E.; Santopuoli, G.; Binder, F.; del Río, M.; La Porta, N.; Kluvankova, T.; Lesinski, J.; Motta, R.; Pach, M.; Panzacchi, P.; et al. What Is Climate-Smart Forestry? A Definition from a Multinational Collaborative Process Focused on Mountain Regions of Europe. Ecosyst. Serv. 2020, 43, 101113. [Google Scholar] [CrossRef]
- Huang, D.; Boxin, O.U.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Stevanato, R.; Fabris, S.; Momo, F. New enzymatic method for the determination of total phenolic content in tea and wine. J. Agric. Food Chem. 2004, 52, 6287–6293. [Google Scholar] [CrossRef]
- Gil-Pelegrín, E.; Javier, J.; Domingo, P.P.; Editors, S.-K. Oaks Physiological Ecology. In Exploring the Functional Diversity of Genus Quercus L.; Springer: Cham, Swizerland, 2017. [Google Scholar]
- Ghanbary, E.; Tabari Kouchaksaraei, M.; Zarafshar, M.; Bader, K.F.M.; Mirabolfathy, M.; Ziaei, M. Differential physiological and biochemical responses of Quercus infectoria and Q. libani to drought and charcoal disease. Physiol. Plant. 2020, 168, 876–892. [Google Scholar] [CrossRef]
- Cotrozzi, L.; Remorini, D.; Pellegrini, E.; Landi, M.; Massai, R.; Nali, C.; Guidi, L.; Lorenzini, G. Variations in physiological and biochemical traits of Oak seedlings grown under drought and ozone stress. Physiol. Plant. 2016, 157, 69–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cotrozzi, L.; Remorini, D.; Pellegrini, E.; Guidi, L.; Lorenzini, G.; Massai, R.; Nali, C.; Landi, M. Cross-talk between physiological and metabolic adjustments adopted by Quercus cerris to mitigate the effects of severe drought and realistic future ozone concentrations. Forests 2017, 8, 148. [Google Scholar] [CrossRef] [Green Version]
- Tausz, M.; Šircelj, H.; Grill, D. The glutathione system as a stress marker in plant ecophysiology: Is a stress response concept valid? J. Exp. Bot. 2004, 55, 1955–1962. [Google Scholar] [CrossRef] [PubMed]
- Vastag, E.; Cocozza, C.; Orlović, S.; Kesić, L.; Kresoja, M.; Stojnić, S. Half-sib lines of pedunculate oak (Quercus robur L.) respond differently to drought through biometrical, anatomical and physiological traits. Forests 2020, 11, 153. [Google Scholar] [CrossRef] [Green Version]
- Stojnić, S.; Kovačević, B.; Kebert, M.; Vaštag, E.; Bojović, M.; Neđić, M.S.; Orlovic, S. The use of physiological, biochemical and morpho-anatomical traits in tree breeding for improved water-use efficiency of Quercus robur L. For. Syst. 2019, 28, 5. [Google Scholar] [CrossRef]
- Dascaliuc, A.; Ralea, T.; Cuza, P. Influence of heat shock on chlorophyll fluorescence of white oak (Quercus pubescens Willd.) leaves. Photosynthetica 2007, 45, 469–471. [Google Scholar] [CrossRef]
- Rapparini, F.; Tam, Y.Y.; Cohen, J.D.; Slovin, J.P. Indole-3-Acetic Acid Metabolism in Lemna Gibba Undergoes Dynamic Changes in Response to Growth Temperature. Plant Physiol. 2002, 128, 1410–1416. [Google Scholar] [CrossRef] [Green Version]
- Blum, A. Osmotic Adjustment Is a Prime Drought Stress Adaptive Engine in Support of Plant Production. Plant Cell Environ. 2017, 40, 4–10. [Google Scholar] [CrossRef]
- Deligöz, A.; Bayar, E. Drought stress responses of seedlings of two oak species (Quercus cerris and Quercus robur). Turk. J. Agric. For. 2018, 42, 114–123. [Google Scholar] [CrossRef]
- Xiong, S.; Wang, Y.; Chen, Y.; Gao, M.; Zhao, Y.; Wu, L. Effects of drought stress and rehydration on physiological and biochemical properties of four oak species in China. Plants 2022, 11, 679. [Google Scholar] [CrossRef]
- Trossat, C.; Rathinasabapathi, B.; Weretilnyk, E.A.; Shen, T.L.; Huang, Z.H.; Gage, D.A.; Hanson, A.D. Salinity promotes accumulation of 3-dimethylsulfoniopropionate and its precursor S-methylmethionine in chloroplasts. Plant Physiol. 1998, 116, 165–171. [Google Scholar] [CrossRef] [Green Version]
- Haworth, M.; Catola, S.; Marino, G.; Brunetti, C.; Michelozzi, M.; Riggi, E.; Avola, G.; Cosentino, S.L.; Loreto, F.; Centritto, M. Moderate Drought Stress Induces Increased Foliar Dimethylsulphoniopropionate (DMSP) Concentration and Isoprene Emission in Two Contrasting Ecotypes of Arundo Donax. Front. Plant Sci. 2017, 8, 1016. [Google Scholar] [CrossRef]
- Ausma, T.; Kebert, M.; Stefels, J.; de Kok, L.J. DMSP: Occurrence in Plants and Response to Salinity in Zea mays. In Sulfur Metabolism in Higher Plants-Fundamental, Environmental and Agricultural Aspects; Springer: New York, NY, USA, 2017; pp. 87–91. [Google Scholar] [CrossRef]
- Catola, S.; Ganesha, S.D.K.; Calamai, L.; Loreto, F.; Ranieri, A.; Centritto, M. Headspace-Solid Phase Microextraction Approach for Dimethylsulfoniopropionate Quantification in Solanum lycopersicum Plants Subjected to Water Stress. Front. Plant Sci. 2016, 7, 1257. [Google Scholar] [CrossRef] [Green Version]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef]
- Hossain, M.A.; Li, Z.G.; Hoque, T.S.; Burritt, D.J.; Fujita, M.; Munné-Bosch, S. Heat or Cold Priming-Induced Cross-Tolerance to Abiotic Stresses in Plants: Key Regulators and Possible Mechanisms. Protoplasma 2018, 255, 399–412. [Google Scholar] [CrossRef]
- Sakamoto, A.; Murata, N. The role of glycine betaine in the protection of plants from stress: Clues from transgenic plants. Plant Cell Environ. 2002, 25, 163–171. [Google Scholar] [CrossRef]
- Mulholland, M.M.; Otte, M.L. The effects of nitrogen supply and salinity on DMSP, glycine betaine and proline concentrations in leaves of Spartina anglica. Aquat. Bot. 2002, 72, 193–200. [Google Scholar] [CrossRef]
- Thariath, D.V.; Divakaran, D.; Chenicherry, S. Influence of salinity on the dimethylsulphoniopropionate production from Prymnesium simplex. Sustain. Environ. Res. 2019, 29, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Ionescu, M.; Edu, E.M.; Mihalache, M.; Ciuvat, L.A. Study on carbon, nitrogen and sulfur in litter Quercus robur, Tilia sp., Carpinus betulas, and Fagus sylvatica. Sci. Pap.-Ser. A Agron. 2013, 56, 48–50. [Google Scholar]
- Smirnof, N. The role of active oxygen in the response of plants to water deficit and desiccation. New Phytol. 1993, 125, 27–58. [Google Scholar] [CrossRef]
- 80. Cherit-Hacid, F.; Derridij, A.; Moulti-Mati, F.; Mati, A. Drought stress effect on some biochemical and physiological parameters; accumulation on total polyphenols and flavonoids in leaves of two provenance seedling Pistacia lentiscus. Int. J. Res. Appl. Nat. Soc. Sci. 2015, 3, 127–138. [Google Scholar]
- Schwanz, P.; Polle, A. Differential stress responses of antioxidative systems to drought in pendunculate oak (Quercus robur) and maritime pine (Pinus pinaster) grown under high CO2 concentrations. J. Exp. Bot. 2001, 52, 133–143. [Google Scholar] [CrossRef]
- Madritsch, S.; Wischnitzki, E.; Kotrade, P.; Ashoub, A.; Burg, A.; Fluch, S.; Brüggemann, W.; Sehr, E.M. Elucidating drought stress tolerance in European oaks through cross-species transcriptomics. G3-Genes Genom Genet. 2019, 10, 3181–3199. [Google Scholar] [CrossRef] [Green Version]
- Jamieson, M.A.; Schwartzberg, E.G.; Raffa, K.F.; Reich, P.B.; Lindroth, R.L. Experimental Climate Warming Alters Aspen and Birch Phytochemistry and Performance Traits for an Outbreak Insect Herbivore. Glob. Chang. Biol. 2015, 21, 2698–2710. [Google Scholar] [CrossRef]
- Ahmed, Z.B.; Yousfi, M.; Viaene, J.; Dejaegher, B.; Demeyer, K.; Mangelings, D.; Heyden, Y. Vander Seasonal, Gender and Regional Variations in Total Phenolic, Flavonoid, and Condensed Tannins Contents and in Antioxidant Properties from Pistacia atlantica ssp. Leaves. Pharm. Biol. 2017, 55, 1185–1194. [Google Scholar] [CrossRef] [Green Version]
- Munné-Bosch, S. The role of α-tocopherol in plant stress tolerance. J. Plant Physiol. 2005, 162, 743–748. [Google Scholar] [CrossRef]
- Saunier, A.; Ormeño, E.; Havaux, M.; Wortham, H.; Ksas, B.; Temime-Roussel, B.; Blande, J.D.; Lecareux, C.; Mévy, J.P.; Bousquet-Mélou, A. Resistance of Native Oak to Recurrent Drought Conditions Simulating Predicted Climatic Changes in the Mediterranean Region. Plant Cell Environ. 2018, 41, 2299–2312. [Google Scholar] [CrossRef] [Green Version]
- Monson, R.K.; Jones, R.T.; Rosenstiel, T.N.; Schnitzler, J.P. Why only some plants emit isoprene. Plant Cell Environ. 2013, 36, 503–516. [Google Scholar] [CrossRef]
- Sharkey, T.D.; Yeh, S. Isoprene emission from plants. Annu. Rev. Plant Biol. 2001, 52, 407–436. [Google Scholar] [CrossRef]
- Peñuelas, J.; Munné-Bosch, S. Isoprenoids: An Evolutionary Pool for Photoprotection. Trends Plant Sci. 2005, 10, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Kesselmeier, J.; Staudt, M. Biogenic Volatile Organic Compounds (VOC): An Overview on Emission, Physiology and Ecology. J. Atmos. Chem. 1999, 33, 23–88. [Google Scholar] [CrossRef]
- Pollastri, S.; Baccelli, I.; Loreto, F. Isoprene: An Antioxidant Itself or a Molecule with Multiple Regulatory Functions in Plants? Antioxidants 2021, 10, 684. [Google Scholar] [CrossRef] [PubMed]
- Vickers, C.E.; Gershenzon, J.; Lerdau, M.T.; Loreto, F. A unified mechanism of action for volatile isoprenoids in plant abiotic stress. Nat. Chem. Biol. 2009, 5, 283–291. [Google Scholar] [CrossRef]
- Li, Z.; Ratliff, E.A.; Sharkey, T.D. Effect of temperature on postillumination isoprene emission in oak and poplar. Plant Physiol. 2011, 155, 1037–1046. [Google Scholar] [CrossRef] [Green Version]
- Behnke, K.; Kleist, E.; Uerlings, R.; Wildt, J.; Rennenberg, H.; Schnitzler, J.P. RNAi-Mediated Suppression of Isoprene Biosynthesis in Hybrid Poplar Impacts Ozone Tolerance. Tree Physiol. 2009, 29, 725–736. [Google Scholar] [CrossRef]
- Monson, R.K.; Weraduwage, S.M.; Rosenkranz, M.; Schnitzler, J.P.; Sharkey, D.T. Leaf Isoprene Emission as a Trait That Mediates the Growth-Defense Tradeoff in the Face of Climate Stress. Oecologia 2021, 197, 885–902. [Google Scholar] [CrossRef]
- De Diego, N.; Saiz-Fernández, I.; Rodríguez, J.L.; Pérez-Alfocea, P.; Sampedro, M.C.; Barrio, R.J.; Lacuesta, M.; Moncaleán, P. Metabolites and hormones are involved in the intraspecific variability of drought hardening in radiata pine. J. Plant Physiol. 2015, 188, 64–71. [Google Scholar] [CrossRef]
- Müller, M.; Munné-Bosch, S. Hormonal impact on photosynthesis and photoprotection in plants. Plant Physiol. 2021, 185, 1500–1522. [Google Scholar] [CrossRef]
- Müller, M.; Munné-Bosch, S. Ethylene Response Factors: A Key Regulatory Hub in Hormone and Stress Signaling. Plant Physiol. 2015, 169, 32–41. [Google Scholar] [CrossRef] [Green Version]
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant Hormone-Mediated Regulation of Stress Responses. BMC Plant Biol. 2016, 16, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Long, A.; Zhang, J.; Yang, L.-T.; Ye, X.; Lai, N.-W.; Tan, L.-L.; Lin, D.; Chen, L.-S. Effects of Low PH on Photosynthesis, Related Physiological Parameters, and Nutrient Profiles of Citrus. Front. Plant Sci. 2017, 8, 185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nardini, A.; Lo Gullo, M.A.; Salleo, S. Competitive Strategies for Water Availability in Two Mediterranean Quercus Species. Plant Cell Environ. 1999, 22, 109–116. [Google Scholar] [CrossRef]
- Aasamaa, K.; Sõber, A.; Hartung, W.; Niinemets, Ü. Rate of stomatal opening, shoot hydraulic conductance and photosynthetic characteristics in relation to leaf abscisic acid concentration in six temperate deciduous trees. Tree Physiol. 2002, 22, 267–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, F.; Zhan, D.; Xu, L.; Han, L.; Zhang, X. Antioxidant and Hormone Responses to Heat Stress in Two Kentucky Bluegrass Cultivars Contrasting in Heat Tolerance. J. Am. Soc. Hortic. Sci. 2014, 139, 587–596. [Google Scholar] [CrossRef] [Green Version]
- Rasulov, B.; Bichele, I.; Laisk, A.; Niinemets, Ü. Competition between Isoprene Emission and Pigment Synthesis during Leaf Development in Aspen. Plant Cell Environ. 2014, 37, 724–741. [Google Scholar] [CrossRef] [Green Version]
- Munné-Bosch, S.; Peñuelas, J.; Asensio, D.; Llusià, J. Airborne Ethylene May Alter Antioxidant Protection and Reduce Tolerance of Holm Oak to Heat and Drought Stress. Plant Physiol. 2004, 136, 2937–2947. [Google Scholar] [CrossRef] [Green Version]
- Weiss, D.; Ori, N.; Smith, R.H. Update on Cross Talk between Gibberellin and Other Hormones Mechanisms of Cross Talk between Gibberellin and Other Hormones. Plant Physiol. 2007, 144, 1240–1246. [Google Scholar] [CrossRef] [Green Version]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid Determination of Free Proline for Water-Stress Studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Grieve, C.M.; Grattan, S.R. Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil 1983, 70, 303–307. [Google Scholar] [CrossRef]
- Stefels, J.; Carnat, G.; Dacey, J.W.H.; Goossens, T.; Elzenga, J.T.M.; Tison, J.L. The Analysis of Dimethylsulfide and Dimethylsulfoniopropionate in Sea Ice: Dry-Crushing and Melting Using Stable Isotope Additions. Mar. Chem. 2012, 128–129, 34–43. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the Thiobarbituric Acid-Reactive-Substances Assay for Estimating Lipid Peroxidation in Plant Tissues Containing Anthocyanin and Other Interfering Compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- De Kok, L.J.; Buwalda, F.; Bosma, W. Determination of cysteine and its accumulation in spinach leaf tissue upon exposure to excess sulfur. J. Plant Physiol. 1988, 133, 502–505. [Google Scholar] [CrossRef]
- Miller, N.J.; Rice-Evans, C.A. Factors Influencing the Antioxidant Activity Determined by the ABTS•+ Radical Cation Assay. Free Radic. Res. 1997, 26, 195–199. [Google Scholar] [CrossRef]
- Arnao, M.B. Some Methodological Problems in the Determination of Antioxidant Activity Using Chromogen Radicals: A Practical Case. Trends Food Sci Technol. 2000, 11, 419–421. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.O.; Jeong, S.W.; Lee, C.Y. Antioxidant Capacity of Phenolic Phytochemicals from Various Cultivars of Plums. Food Chem. 2003, 81, 321–326. [Google Scholar] [CrossRef]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar] [CrossRef] [Green Version]
- Porter, L.J.; Hrstich, L.N.; Chan, B.G. The Conversion of Procyanidins and Prodelphinidins to Cyanidin and Delphinidin. Phytochemistry 1985, 25, 223–230. [Google Scholar] [CrossRef] [Green Version]
- Karthikeyan, R.; Kumaravel, S. Study on Phenolic content, Antioxidant Activity and CHNS elemental analysis of Amorphophallus sylvaticus. Int. J. Agric. Life Sci. 2016, 2, 12–17. [Google Scholar]
- Cohen, J.D. Convenient Apparatus for the Generation of Small Amounts of Diazomethane. J. Chromatogr. A 1984, 303, 193–196. [Google Scholar] [CrossRef]
- Ruiz, K.B.; Rapparini, F.; Bertazza, G.; Silva, H.; Torrigiani, P.; Biondi, S. Comparing salt-induced responses at the transcript level in a salares and coastal-lowlands landrace of quinoa (Chenopodium quinoa Willd). Environ. Exp. Bot. 2017, 139, 127–142. [Google Scholar] [CrossRef]
- Cohen, J.D.; Baldi, J.P.; Slovin, S.P. A new internal standard for quantitative mass spectral analysis of indole-3-acetic acid in plants. Plant Physiol. 1987, 80, 14–19. [Google Scholar] [CrossRef] [Green Version]









| Plant Species | Plant Age | Growth Conditions | Stress Design | Response to Drought | Response to High Temperature | Literature |
|---|---|---|---|---|---|---|
| Q. robur | 10 weeks | Potted plants under controlled conditions | Drought: withholding water for about 21 days | ↓ Redox ratio of ascorbate and glutathione ↑ MDA ↓ Carotenoids | Schwanz and Polle, 2001 | |
| Q. robur | 5 years | Potted plants in greenhouse | Drought: withholding water to reach soil moisture level of 13% | ↑ Carbohydrates (hexoses) and polyols ↑ Proline and Proline derivatives ‖ Glycine betaine | Spieß et al., 2012 | |
| Q. robur | 3–5 years | Potted plants under controlled conditions | Drying-rewetting cycles (reduced irrigation for about 20 and 30 days) High temperature (+1/2 °C compared to controlled conditions) | ↑ Proline, GABA ↑ Glutathione ‖ Total and Reduced Ascorbate ‖ Cysteine and γ-glutamyl cysteine | ‖ Proline, GABA ‖ Glutathione ‖ Total and Reduced Ascorbate ‖ Cysteine and γ-glutamyl cysteine | Hu et al., 2013 |
| Q. robur | 3–5 years | Potted plants under controlled conditions | Drying-rewetting cycles (reduced irrigation for about 20 and 30 days) High temperature (+1/2 °C compared to controlled conditions) | ↑ Total and specific amino acids-N and Glutamine | ‖ Total and specific amino acids-N | Hu et al., 2015 |
| Q. cerris | 3 years | Potted plants in greenhouse under controlled conditions | Drought (daily irrigation with 30% effective evapotranspiration for 2 weeks) | ↑ Proline, MDA ↑ Chlorophylls ‖ Carotenoids (xantophylls) | Cotrozzi et al., 2015 | |
| Q. cerris | 3 years | Potted plants in greenhouse under controlled conditions | Drought (daily irrigation with 20% effective evapotranspiration for 2 weeks) | ↑ Proline, MDA ↑ Carotenoids, chlorophylls, β-carotene, α-tocopherol, ABA ‖ Carbohydrates (hexoses) | Cotrozzi et al., 2016 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kebert, M.; Vuksanović, V.; Stefels, J.; Bojović, M.; Horák, R.; Kostić, S.; Kovačević, B.; Orlović, S.; Neri, L.; Magli, M.; et al. Species-Level Differences in Osmoprotectants and Antioxidants Contribute to Stress Tolerance of Quercus robur L., and Q. cerris L. Seedlings under Water Deficit and High Temperatures. Plants 2022, 11, 1744. https://doi.org/10.3390/plants11131744
Kebert M, Vuksanović V, Stefels J, Bojović M, Horák R, Kostić S, Kovačević B, Orlović S, Neri L, Magli M, et al. Species-Level Differences in Osmoprotectants and Antioxidants Contribute to Stress Tolerance of Quercus robur L., and Q. cerris L. Seedlings under Water Deficit and High Temperatures. Plants. 2022; 11(13):1744. https://doi.org/10.3390/plants11131744
Chicago/Turabian StyleKebert, Marko, Vanja Vuksanović, Jacqueline Stefels, Mirjana Bojović, Rita Horák, Saša Kostić, Branislav Kovačević, Saša Orlović, Luisa Neri, Massimiliano Magli, and et al. 2022. "Species-Level Differences in Osmoprotectants and Antioxidants Contribute to Stress Tolerance of Quercus robur L., and Q. cerris L. Seedlings under Water Deficit and High Temperatures" Plants 11, no. 13: 1744. https://doi.org/10.3390/plants11131744