Distribution of the Order Lampriformes in the Mediterranean Sea with Notes on Their Biology, Morphology, and Taxonomy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Biological and Ecological Features of Lampriformes
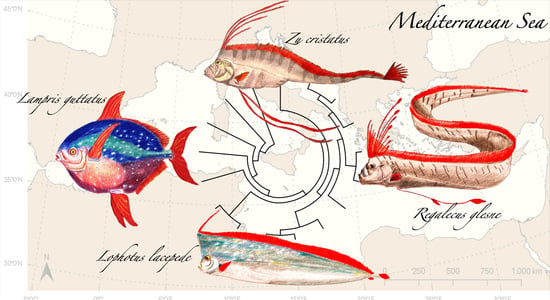
3. Essential Systematics and Phylogeny of the Group
Lampriformes Phylogenetic Relationships Based on mt-COI Sequences
4. Distribution of Lampriformes Orders in the Mediterranean Sea
4.1. Lampridae (Gill, 1862)
4.2. Lophotidae (Bonaparte, 1845)
4.3. Radiicephalidae (Osorio, 1917)
4.4. Regalecidae (Gill, 1884)
4.5. Trachipteridae (Swainson, 1839)
4.6. Veliferidae (Bleeker, 1859)
5. Final Considerations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Olney, J.E. Order Lampriformes. In FAO Species Identification Guide for Fishery Purposes. The Living Marine Resources of the Western Central Pacific. Volume 3: Batoid Fishes, Chimaeras and Bony Fishes Part 1 (Elopidae to Linophrynidae); Carpenter, K.E., Niem, V.H., Eds.; FAO: Rome, Italy, 1999; pp. 952–959. ISBN 92-5-104302-7. [Google Scholar]
- Nelson, J.S.; Grande, T.C.; Wilson, M.V.H. Fish of the World; Wiley: Hoboken, NJ, USA, 2016; pp. 1–752. [Google Scholar]
- Falsone, F.; Geraci, M.L.; Scannella, D.; Okpala, C.O.R.; Giusto, G.B.; Bosch-Belmar, M.; Gancitano, S.; Bono, G. Occurrence of two rare species from order lampriformes: Crestfish Lophotus lacepede (Giorna, 1809) and scalloped ribbonfish Zu cristatus (Bonelli, 1819) in the northern coast of sicily, Italy. Acta Adriat. 2017, 58, 137–145. [Google Scholar] [CrossRef]
- Quigley, D.T. Sherkin Comment; Sherkin Island Marine Station: Cork, Ireland, 2012; p. 12. [Google Scholar]
- Garibaldi, F. By-catch in the mesoplagic swordfish longline fishery in the ligurian sea (Western Mediterranean). ICCAT 2015, 71, 1495–1498. [Google Scholar]
- Dulčić, J. First record of scalloped ribbon fish, Zu cristatus (Pisces: Trachipteridae), eggs in the Adriatic Sea. J. Plankton Res. 2002, 24, 1245–1246. [Google Scholar] [CrossRef]
- Moritz, T.; Stümer, D.; Jakobsen, K.; Jakobsen, J. Observations on two live specimens of Trachipterus arcticus (Lampriformes: Trachipteridae) from the Azores. Cybium 2015, 39, 78–80. [Google Scholar]
- Dragicevic, B.; Pallaoro, A.; Grgicevic, R.; Lipej, L.; Dulcic, J. On the occurrence of early life stage of the king of herrings, Regalecus glesne (Actinopterygii: Lampriformes: Regalecidae), in the Adriatic Sea. Acta Ichthyol. Piscat. 2011, 41, 251. [Google Scholar] [CrossRef]
- de Busserolles, F.; Fogg, L.; Cortesi, F.; Marshall, J. The exceptional diversity of visual adaptations in deep-sea teleost fishes. Semin. Cell Dev. Biol. 2020, 106, 20–30. [Google Scholar] [CrossRef]
- Weber, A.A.T.; Hugall, A.F.; O’Hara, T.D. Convergent evolution and structural adaptation to the deep ocean in the protein-folding chaperonin CCTα. Genome Biol. Evol. 2020, 12, 1929–1942. [Google Scholar] [CrossRef]
- Lupše, N.; Cortesi, F.; Freese, M.; Marohn, L.; Pohlman, J.-D.; Wysujack, K.; Hanel, R.; Musilova, Z. Visual gene expression reveals a cone to rod developmental progression in deep-sea fishes. Mol. Biol. Evol. 2021, 38, 5664–5677. [Google Scholar] [CrossRef]
- Brown, A.; Thatje, S. The effects of changing climate on faunal depth distributions determine winners and losers. Glob. Chang. Biol. 2015, 21, 173–180. [Google Scholar] [CrossRef] [Green Version]
- Wiley, E.O.; David Johnson, G.; Wheaton Dimmick, W. The interrelationships of Acanthomorph fishes: A total evidence approach using molecular and morphological data. Biochem. Syst. Ecol. 2000, 28, 319–350. [Google Scholar] [CrossRef]
- Davesne, D.; Friedman, M.; Barriel, V.; Lecointre, G.; Janvier, P.; Gallut, C.; Otero, O. Early fossils illuminate character evolution and interrelationships of Lampridiformes (Teleostei, Acanthomorpha). Zool. J. Linn. Soc. 2014, 172, 475–498. [Google Scholar] [CrossRef] [Green Version]
- Miya, M.; Holcroft, N.I.; Satoh, T.P.; Yamaguchi, M.; Nishida, M.; Wiley, E.O. Mitochondrial genome and a nuclear gene indicate a novel phylogenetic position of deep-sea tube-eye fish (Stylephoridae). Ichthyol. Res. 2007, 54, 323–332. [Google Scholar] [CrossRef]
- Miya, M.; Kawaguchi, A.; Nishida, M. Mitogenomic exploration of higher teleostean phylogenies: A case study for moderate-scale evolutionary genomics with 38 newly determined complete mitochondrial DNA sequences. Mol. Biol. Evol. 2001, 18, 1993–2009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macali, A.; Semenov, A.; de Mendoza, F.P.; Dinoi, A.; Bergami, E.; Tiralongo, F. Relative influence of environmental factors on biodiversity and behavioural traits of a rare mesopelagic fish, Trachipterus trachypterus (gmelin, 1789), in a continental shelf front of the Mediterranean Sea. J. Mar. Sci. Eng. 2020, 8, 581. [Google Scholar] [CrossRef]
- Pinsky, M.L.; Worm, B.; Fogarty, M.J.; Sarmiento, J.L.; Levin, S.A. Marine taxa track local climate velocities. Science 2013, 341, 1239–1242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koenigstein, S.; Mark, F.C.; Gößling-Reisemann, S.; Reuter, H.; Poertner, H.O. Modelling climate change impacts on marine fish populations: Process-based integration of ocean warming, acidification and other environmental drivers. Fish Fish. 2016, 17, 972–1004. [Google Scholar] [CrossRef] [Green Version]
- Heath, M.R.; Neat, F.C.; Pinnegar, J.K.; Reid, D.G.; Sims, D.W.; Wright, P.J. Review of climate change impacts on marine fish and shellfish around the UK and Ireland. Aquat. Conserv. Mar. Freshw. Ecosyst. 2012, 22, 337–367. [Google Scholar] [CrossRef]
- Rinaldi, A.; Montalto, V.; Manganaro, A.; Mazzola, A.; Mirto, S.; Sanfilippo, M.; Sarà, G. Predictive mechanistic bioenergetics to model habitat suitability ofshellfish culture in coastal lakes. Estuar. Coast. Shelf Sci. 2014, 144, 89–98. [Google Scholar] [CrossRef]
- Albano, M.; Panarello, G.; Di Paola, D.; D’Angelo, G.; Granata, A.; Savoca, S.; Capillo, G. The mauve stinger Pelagia noctiluca (Cnidaria, Scyphozoa) plastics contamination, the Strait of Messina case. Int. J. Environ. Stud. 2021, 78, 977–982. [Google Scholar] [CrossRef]
- Savoca, S.; Grifó, G.; Panarello, G.; Albano, M.; Giacobbe, S.; Capillo, G.; Spanó, N.; Consolo, G. Modelling prey-predator interactions in Messina beachrock pools. Ecol. Modell. 2020, 434, 109206. [Google Scholar] [CrossRef]
- Tiralongo, F.; Lillo, A.O.; Tibullo, D.; Tondo, E.; Martire, C.L.; D’Agnese, R.; Macali, A.; Mancini, E.; Giovos, I.; Coco, S.; et al. Monitoring uncommon and non-indigenous fishes in Italian waters: One year of results for the AlienFish project. Reg. Stud. Mar. Sci. 2019, 28, 100606. [Google Scholar] [CrossRef]
- Coco, S.; Roncarati, A.; Tiralongo, F.; Felici, A. Meridionalization as a Possible Resource for Fisheries: The Case Study of Caranx rhonchus Geoffroy Saint-Hilaire, 1817, in Southern Italian Waters. J. Mar. Sci. Eng. 2022, 10, 274. [Google Scholar] [CrossRef]
- Capillo, G.; Panarello, G.; Savoca, S.; Sanfilippo, M.; Albano, M.; Volsi, R.L.; Consolo, G.; Spanò, N. Intertidal ponds of messina’s beachrock faunal assemblage, evaluation of ecosystem dynamics and communities’ interactions. Atti Della Accad. Peloritana Dei Pericolanti-Cl. Di Sci. Fis. Mat. E Nat. 2018, 96, A41–A416. [Google Scholar] [CrossRef]
- Perry, A.L.; Low, P.J.; Ellis, J.R.; Reynolds, J.D. Ecology: Climate change and distribution shifts in marine fishes. Science 2005, 308, 1912–1915. [Google Scholar] [CrossRef]
- Brown, A.; Thatje, S. Explaining bathymetric diversity patterns in marine benthic invertebrates and demersal fishes: Physiological contributions to adaptation of life at depth. Biol. Rev. 2014, 89, 406–426. [Google Scholar] [CrossRef]
- Crozier, L.G.; Hendry, A.P.; Lawson, P.W.; Quinn, T.P.; Mantua, N.J.; Battin, J.; Shaw, R.G.; Huey, R.B. Potential responses to climate change in organisms with complex life histories: Evolution and plasticity in Pacific salmon. Evol. Appl. 2008, 1, 252–270. [Google Scholar] [CrossRef]
- Caves, E.M.; Johnsen, S. The sensory impacts of climate change: Bathymetric shifts and visually mediated interactions in aquatic species. Proc. R. Soc. B Biol. Sci. 2021, 288, 20210396. [Google Scholar] [CrossRef]
- Buran, B.N.; Deng, X.; Popper, A.N. Structural variation in the inner ears of four deep-sea elopomorph fishes. J. Morphol. 2005, 265, 215–225. [Google Scholar] [CrossRef]
- Gibbs, A.; Somero, G.N. Pressure adaptation of Na+/K+-ATPase in gills of marine teleosts. J. Exp. Biol. 1989, 143, 475–492. [Google Scholar] [CrossRef]
- Morita, T. High-pressure adaptation of muscle proteins from deep-sea fishes, Coryphaenoides yaquinae and C. armatus. Ann. N. Y. Acad. Sci. 2010, 1189, 91–94. [Google Scholar] [CrossRef]
- Wakai, N.; Takemura, K.; Morita, T.; Kitao, A. Mechanism of deep-sea fish a-actin pressure tolerance investigated by molecular dynamics simulations. PLoS ONE 2014, 9, e85852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pörtner, H.O.; Knust, R. Climate change affects marine fishes through the oxygen limitation of thermal tolerance. Science 2007, 315, 95–97. [Google Scholar] [CrossRef] [PubMed]
- Pörtner, H.O.; Peck, M.A. Climate change effects on fishes and fisheries: Towards a cause-and-effect understanding. J. Fish Biol. 2010, 77, 1745–1779. [Google Scholar] [CrossRef] [PubMed]
- Spanò, N.; Domenico, E. De Biodiversity in Central Mediterranean Sea. In Mediterranean Identities—Environment, Society, Culture; IntechOpen: London, UK, 2017. [Google Scholar]
- Renzi, M.; Provenza, F.; Pignattelli, S.; Cilenti, L.; Specchiulli, A.; Pepi, M. Mediterranean coastal lagoons: The importance of monitoring in sediments the biochemical composition of organic matter. Int. J. Environ. Res. Public Health 2019, 16, 3466. [Google Scholar] [CrossRef] [Green Version]
- Sanfilippo, M.; Albano, M.; Manganaro, A.; Capillo, G.; Spanò, N.; Savoca, S. Spatiotemporal Organic Carbon Distribution in the Capo Peloro Lagoon (Sicily, Italy) in Relation to Environmentally Sustainable Approaches. Water 2022, 14, 108. [Google Scholar] [CrossRef]
- Manganaro, A.; Pulicanò, G.; Sanfilippo, M. Temporal evolution of the area of Capo Peloro (Sicily, Italy) from pristine site into urbanized area. Transit. Waters Bull. 2011, 5, 23–31. [Google Scholar] [CrossRef]
- Spanò, N.; Di Paola, D.; Albano, M.; Manganaro, A.; Sanfilippo, M.; D’Iglio, C.; Capillo, G.; Savoca, S. Growth performance and bioremediation potential of Gracilaria gracilis (Steentoft, L.M. Irvine & Farnham, 1995). Int. J. Environ. Stud. 2021, 79, 748–760. [Google Scholar] [CrossRef]
- Sarà, G.; Romano, C.; Widdows, J.; Staff, F.J. Effect of salinity and temperature on feeding physiology and scope for growth of an invasive species (Brachidontes pharaonis—Mollusca: Bivalvia) within the Mediterranean sea. J. Exp. Mar. Bio. Ecol. 2008, 363, 130–136. [Google Scholar] [CrossRef]
- Petrocelli, A.; Cecere, E.; Verlaque, M. Alien marine macrophytes in transitional water systems: New entries and reappearances in a Mediterranean coastal basin. BioInvasions Rec. 2013, 2, 177–184. [Google Scholar] [CrossRef]
- Zenetos, A.; Akel, E.H.K.; Apostolidis, C.; Bilecenoglu, M.; Bitar, G.; Buchet, V.; Chalari, N.; Corsini-Foka, M.; Crocetta, F.; Dogrammatzi, A.; et al. New mediterranean biodiversity records (April 2015). Mediterr. Mar. Sci. 2015, 16, 266–284. [Google Scholar] [CrossRef]
- Pérez-Ruzafa, A.; Quispe-Becerra, J.I.; Garcĩa-Charton, J.A.; Marcos, C. Composition, structure and distribution of the ichthyoplankton in a Mediterranean coastal lagoon. J. Fish Biol. 2004, 64, 202–218. [Google Scholar] [CrossRef]
- Sinopoli, M.; Pipitone, C.; Campagnuolo, S.; Campo, D.; Castriota, L.; Mostarda, E.; Andaloro, F. Diet of young-of-the-year bluefin tuna, Thunnus thynnus (Linnaeus, 1758), in the southern Tyrrhenian (Mediterranean) Sea. J. Appl. Ichthyol. 2004, 20, 310–313. [Google Scholar] [CrossRef]
- Elliott, M.; Day, J.W.; Ramachandran, R.; Wolanski, E.; Fang, Q.; Sheehan, M.R.; Seen, A.J.; Ellison, J.C. A Synthesis: What Is the Future for Coasts, Estuaries, Deltas and Other Transitional Habitats in 2050 and Beyond? In Coasts and Estuaries The Future; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–28. [Google Scholar] [CrossRef]
- Underkoffler, K.E.; Luers, M.A.; Hyde, J.R.; Craig, M.T. A taxonomic review of Lampris guttatus (Brünnich 1788) (Lampridiformes; Lampridae) with descriptions of three new species. Zootaxa 2018, 4413, 551–565. [Google Scholar] [CrossRef] [PubMed]
- Psomadakis, P.N.; Bottaro, M.; Vacchi, M. On two large specimens of Zu cristatus (Trachipteridae) from the Gulf of Genoa (NW Mediterranean). Available online: https://sfi-cybium.fr/fr/two-large-specimens-zu-cristatus-trachipteridae-gulf-genoa-nw-mediterranean (accessed on 6 March 2022).
- Olney, J.E. Lampridiformes: Development and relationships. In Ontogeny and systematics of fishes. American Society of Ichthyologists and Herpetologists Special Publication No. 1; Moser, H.G., Richards, W.J., Cohen, D.M., Fahay, M.P., Kendall, A.W., Jr., Richardson, S.L., Eds.; Allen Press: Lawrence, KS, USA, 1984; pp. 368–379. [Google Scholar]
- Wiley, E.O.; Johnson, G.D.; Dimmick, W.W. The Phylogenetic Relationships of Lampridiform Fishes (Teleostei: Acanthomorpha), Based on a Total-Evidence Analysis of Morphological and Molecular Data. Mol. Phylogenet. Evol. 1998, 10, 417–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, J.M. Phylogeny, Ontogeny and Distribution of the Ribbonfishes (Lampridiformes: Trachipteridae); The College of William and Mary: Williamsburg, VA, USA, 2015. [Google Scholar] [CrossRef]
- Palmer, G. Lophotidae. In Fishes of the North-Eastern Atlantic and the Mediterra-Nean; White-head, P.J.P., Bauchot, M.L., Hureau, J.C., Nielsen, J., Tortonese, E., Eds.; Richard Clay (The Chaucer Press) Ltd.: Bungay, UK, 1986; Volume 2, p. 7. Available online: https://www.google.com/search?q=PALMER%2C+G.+1986.+Lophotidae.+In%3A+P.J.P.+White-+head%2C+M.L.+Bauchot%2C+J.C.+Hureau%2C+J.+Nielsen+and+E.+Tortonese+(Editors).+Fishes+of+the+north-eastern+Atlantic+and+the+Mediterra-+nean.+Volume+2.+Richard+Clay+(The+Chau (accessed on 6 March 2022).
- Borme, D.; Voltolina, F. On the occurrence of ribbon fish Trachipterus trachypterus (Gmelin, 1789) in the Gulf of Trieste (Northern Adriatic Sea). Ann. Ser. Hist. Nat. 2006, 16, 181–188. [Google Scholar]
- Garibaldi, F.; Orsi Relini, L. Summer abundance, size, and feeding habits of the blue shark, Prionace glauca, in the pelagic sanctuary of the Ligurian Sea. Biol. Mar. Mediterr. 2000, 7, 324–333. [Google Scholar]
- Tamura, T.; Kubodera, T.; Ohizumi, H.; Isoda, T. Feeding habits of sperm whales and their impact on neon flying squid resources in the western North Pacific. Sc/J09/Jr17 2008, 9, 1–22. [Google Scholar]
- Strona, G.; Palomares, M.L.D.; Bailly, N.; Galli, P.; Lafferty, K.D. Host range, host ecology, and distribution of more than 11 800 fish parasite species. Ecology 2013, 94, 544. [Google Scholar] [CrossRef]
- D’Iglio, C.; Albano, M.; Famulari, S.; Spanò, N.; Rinelli, P.; Savoca, S.; Capillo, G. Basic Intersexuality (Abnormal Hermaphroditism) in the Blackmouth Catshark, Galeus melastomus, (Rafinesque, 1810), from the Southern Tyrrhenian Sea (Central Mediterranean Sea). Fishes 2022, 7, 120. [Google Scholar] [CrossRef]
- Kim, A.R.; Yoon, T.H.; Lee, C.I.; Kang, C.K.; Kim, H.W. Metabarcoding Analysis of Ichthyoplankton in the East/Japan Sea Using the Novel Fish-Specific Universal Primer Set. Front. Mar. Sci. 2021, 8, 614394. [Google Scholar] [CrossRef]
- Ji, H.W.; Yoon, S.C.; Kim, J.K. Taxonomic review of the family Trachipteridae (Lampridiformes) from Korea. Korean J. Ichthyol. 2009, 21, 273–282. [Google Scholar]
- Olney, J.E.; Johnson, G.D.; Baldwin, C.C. Phylogeny of lampridiform fishes. Bull. Mar. Sci. 1993, 52, 137–169. [Google Scholar]
- Sorbini, C.; Sorbini, L. The Cretaceous Fishes of Nardò, Nardovelifer altipinnis (Teleostei, Lampridiformes, Veliferidae). Stud. e Ric. Sui Giacimenti Terziari Di Bolca 1999, 8, 11–27. [Google Scholar]
- Davies, W.L.; Carvalho, L.S.; Tay, B.H.; Brenner, S.; Hunt, D.M.; Venkatesh, B. Into the blue: Gene duplication and loss underlie color vision adaptations in a deep-sea chimaera, the elephant shark Callorhinchus milii. Genome Res. 2009, 19, 415–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, J.M.; Hilton, E.J. A taxonomic review of the family Trachipteridae (Acanthomorpha: Lampridiformes), with an emphasis on taxa distributed in the western Pacific Ocean. Zootaxa 2021, 5039, 301–351. [Google Scholar] [CrossRef]
- McDowall, R.M.; Stewart, A.L. Further specimens of Agrostlchthys parkeri (teleostei: Regalecidae), with natural history notes. In Proceedings of the 5th Indo-Pacific Fish Conference: Proceedings, New Caledonia, France, 3–8 November 1997. [Google Scholar]
- Honma, Y.; Ushiki, T.; Takeda, M. Histology of the ink tube and its associated organs in a unicornfish, Eumecichthys fiskii (Lampridiformes). Ichthyol. Res. 1999, 46, 19–25. [Google Scholar] [CrossRef]
- Mincarone, M.M.; Lima, A.T.; Soto, J.M.R. Sobre a ocorrência do peixe-fita Trachipterus jacksonensis (Ramsay, 1881) (Lampridiformes, Trachipteridae) na costa brasileira. Mare Magnum 2001, 1, 121–124. [Google Scholar]
- Maina, J.N. Functional morphology of the respiratory organs of the air-breathing fish with particular emphasis on the African catfishes, Clarias mossambicus and C. gariepinus. Acta Histochem. 2018, 120, 613–622. [Google Scholar] [CrossRef]
- Sayed, R.K.A.; Zaccone, G.; Capillo, G.; Albano, M.; Mokhtar, D.M. Structural and Functional Aspects of the Spleen in Molly Fish Poecilia sphenops (Valenciennes, 1846): Synergistic Interactions of Stem Cells, Neurons, and Immune Cells. Biology 2022, 11, 779. [Google Scholar] [CrossRef]
- Mokhtar, D.M.; Sayed, R.K.A.; Zaccone, G.; Albano, M.; Hussein, M.T. Ependymal and Neural Stem Cells of Adult Molly Fish (Poecilia sphenops, Valenciennes, 1846) Brain: Histomorphometry, Immunohistochemical, and Ultrastructural Studies. Cells 2022, 11, 2659. [Google Scholar] [CrossRef]
- Orsi Relini, L.; Palandri, G.; Garibaldi, F.; Cima, C. Longline sworfish fishery in the Ligurian Sea: Eight years of observations on target and by-catch species. Collect. Vol. Sci. Pap. Comm. Conserv. Atl. Tunas 1998, 49, 146–150. [Google Scholar]
- Martín-Cuadrado, A.B.; López-García, P.; Alba, J.C.; Moreira, D.; Monticelli, L.; Strittmatter, A.; Gottschalk, G.; Rodríguez-Valera, F. Metagenomics of the deep Mediterranean, a warm bathypelagic habitat. PLoS ONE 2007, 2, e914. [Google Scholar] [CrossRef] [Green Version]
- Levy, A.; von der Heyden, S.; Floeter, S.R.; Bernardi, G.; Almada, V.C. Phylogeny of Parablennius Miranda Ribeiro, 1915 reveals a paraphyletic genus and recent Indo-Pacific diversification from an Atlantic ancestor. Mol. Phylogenet. Evol. 2013, 67, 1–8. [Google Scholar] [CrossRef]
- Karlsbakk, E.; Kristmundsson, Á.; Albano, M.; Brown, P.; Freeman, M.A. Redescription and phylogenetic position of Myxobolus ‘eglefini’ and Myxobolus platessae n. comb. (Myxosporea), parasites in the cartilage of some North Atlantic marine fishes, with notes on the phylogeny and classification of the Platysporina. Parasitol. Int. 2017, 66, 952–959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Der Laan, R.; Eschmeyer, W.N.; Fricke, R. Family-group names of recent fishes. Zootaxa 2014, 3882, 1–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eschmeyer, W.N.; Ron, F.; Der, L.R. van CAS—Eschmeyer’s Catalog of Fishes. Available online: https://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp (accessed on 5 May 2022).
- Fenchel, T.; Ockelmann, K.W. European register of marine species a check-list of the marine species in Europe and bibliography of guides to their identification. Ophelia 2002, 56, 55. [Google Scholar] [CrossRef]
- Koeda, K.; Ho, H.C. A new tapertail species (family Radiicephalidae; Lampridiformes) from Taiwan, the first confirmed western Pacific Ocean record of the family. Ichthyol. Res. 2019, 66, 207–214. [Google Scholar] [CrossRef]
- Grande, T.; Calvin Borden, W.; Smith, W.L. Limits and relationships of Paracanthopterygii: A molecular framework for evaluating past morphological hypotheses. Mesozoic Fishes 5–Glob. Divers. Evol. 2013, 5, 385–418. [Google Scholar] [CrossRef]
- McEachran, J.D. Fishes (Vertebrata: Pisces) of the Gulf of Mexico. In Gulf of Mexico–Origins, Waters, and Biota. Biodiversity; Texas A&M University Press: College Sation, TX, USA, 2009; pp. 1223–1316. [Google Scholar]
- Davesne, D. A fossil unicorn crestfish (Teleostei, Lampridiformes, Lophotidae) from the Eocene of Iran. PeerJ 2017, 2017, e3381. [Google Scholar] [CrossRef] [Green Version]
- Dettai, A.; Lecointre, G. Further support for the clades obtained by multiple molecular phylogenies in the acanthomorph bush. Comptes Rendus.—Biol. 2005, 328, 674–689. [Google Scholar] [CrossRef]
- Miya, M.; Takeshima, H.; Endo, H.; Ishiguro, N.B.; Inoue, J.G.; Mukai, T.; Satoh, T.P.; Yamaguchi, M.; Kawaguchi, A.; Mabuchi, K.; et al. Major patterns of higher teleostean phylogenies: A new perspective based on 100 complete mitochondrial DNA sequences. Mol. Phylogenet. Evol. 2003, 26, 121–138. [Google Scholar] [CrossRef]
- Carnevale, G. The first fossil ribbonfish (Teleostei, Lampridiformes, Trachipteridae). Geol. Mag. 2004, 141, 573–582. [Google Scholar] [CrossRef]
- Bannikov, A.F. A Review of Fossil Lampridiformes (Teleostei) Finds with a Description of a New Lophotidae Genus and Species from the Oligocène of the Northern Caucasus. Available online: https://www.researchgate.net/publication/266793718_A_Review_of_Fossil_Lampridiformes_Teleostei_Finds_with_a_Description_of_a_New_Lophotidae_Genus_and_Species_from_the_Oligocene_of_the_Northern_Caucasus#fullTextFileContent (accessed on 5 May 2022).
- Bannikov, A.F. A new genus of the family Palaeocentrotidae (Teleostei, Lampridiformes) from the Oligocene of the northern Caucasus and comments on other fossil Veliferoidei. Paleontol. J. 2014, 48, 624–632. [Google Scholar] [CrossRef]
- Carnevale, G.; Bannikov, A.F. A sailfin velifer (Lampridiformes, Veliferidae) fish from the Eocene of Monte Bolca, Italy. Boll. Della Soc. Paleontol. Ital. 2018, 57, 176. [Google Scholar]
- Papazzoni, P.A.; Giusberti, L.; Carnevale, G.; Roghi, G.; Bassi, D.; Zorzin, R. The Bolca Fossil-Lagerstatten: A window into the Eocene World. In Proceedings of the High-Resolution Integrated Biostratigraphy (Larger Foraminifera and Calcareous Nannoplankton) of the Bolca Area (Lessini Mts., Northern Italy); Società Paleontologica Italiana, M., Ed.; Rendiconti della Società Paleontologica Italiana: Modena, Italy, 2014. [Google Scholar]
- Jordan, D.S. The Fossil Fishes of the Miocene of Southern California; Stanford University Press: Stanford, CA, USA, 1927; Volume 9. [Google Scholar]
- Gottfried, M.D.; Fordyce, R.E.; Rust, S. Megalampris keyesi, a giant moonfish (Teleostei, Lampridiformes) from the Late Oligocene of New Zealand. J. Vertebr. Paleéontol. 2006, 26, 544–551. [Google Scholar] [CrossRef]
- Angulo, A.; Lopez-Sanchez, M.I. New records of lampriform fishes (Teleostei: Lampriformes) from the Pacific coast of lower Central America, with comments on the diversity, taxonomy and distribution of the Lampriformes in the eastern Pacific Ocean. Zootaxa 2017, 4236, 573–591. [Google Scholar] [CrossRef]
- Lemoine, F.; Correia, D.; Lefort, V.; Doppelt-Azeroual, O.; Mareuil, F.; Cohen-Boulakia, S.; Gascuel, O. NGPhylogeny.fr: New generation phylogenetic services for non-specialists. Nucleic Acids Res. 2019, 47, W260–W265. [Google Scholar] [CrossRef] [Green Version]
- D’Iglio, C.; Albano, M.; Famulari, S.; Savoca, S.; Panarello, G.; Di Paola, D.; Perdichizzi, A.; Rinelli, P.; Lanteri, G.; Spanò, N.; et al. Intra- and interspecific variability among congeneric Pagellus otoliths. Sci. Rep. 2021, 11, 16315. [Google Scholar] [CrossRef]
- D’iglio, C.; Albano, M.; Tiralongo, F.; Famulari, S.; Rinelli, P.; Savoca, S.; Spanò, N.; Capillo, G. Biological and ecological aspects of the blackmouth catshark (Galeus melastomus rafinesque, 1810) in the southern tyrrhenian sea. J. Mar. Sci. Eng. 2021, 9, 967. [Google Scholar] [CrossRef]
- D’Iglio, C.; Famulari, S.; Albano, M.; Giordano, D.; Rinelli, P.; Capillo, G.; Spanò, N.; Savoca, S. Time-Scale Analysis of Prey Preferences and Ontogenetic Shift in the Diet of European Hake Merluccius merluccius (Linnaeus, 1758) in Southern and Central Tyrrhenian Sea. Fishes 2022, 7, 167. [Google Scholar] [CrossRef]
- Scott, E.O.G. Observations on some Tasmanian fishes: Part XXIX. Pap. Proc.-R. Soc. Tasmania 1983, 117, 167–202. [Google Scholar] [CrossRef]
- Gavagnin, E.P.; EP, G. Considerazioni sulla cattura di uno “Zu cristatus”(bonelli) a Sanremo (osteichthyes trachipteridae). Nat. Milano 1976, 67, 258–261. [Google Scholar]
- Kukuev, E.I. Juvenile Individuals of Opahs (Lampridae) from the Atlantic and Pacific Oceans. Notes on the Systematics and Distribution of Opahs, Including the Description of a New Subgenus, Paralampris subgen. nov. J. Ichthyol. 2021, 61, 182–189. [Google Scholar] [CrossRef]
- Records, I.; Nolan, F.; Quigley, D.T.G. Deal-Fish Trachipterus arcticus (Brünnich, 1788) in Irish Waters: Further Records and a Review of the. Source Irish Nat. J. 1987, 22, 188–189. [Google Scholar]
- Hyde, J.R.; Underkoffler, K.E.; Sundberg, M.A. DNA barcoding provides support for a cryptic species complex within the globally distributed and fishery important opah (Lampris guttatus). Mol. Ecol. Resour. 2014, 14, 1239–1247. [Google Scholar] [CrossRef]
- Polovina, J.J.; Hawn, D.; Abecassis, M. Vertical movement and habitat of opah (Lampris guttatus) in the central North Pacific recorded with pop-up archival tags. Mar. Biol. 2008, 153, 257–267. [Google Scholar] [CrossRef]
- Oelschläger, H.A. Morphologisch-funktionelle Untersuchungen am Geruchsorgan von Lampris guttatus (Brünnich 1788)(Teleostei: Allotriognathi). Zoomorphologie 1976, 85, 89–110. [Google Scholar] [CrossRef]
- Hawn, D.R.; Collette, B.B. What are the maximum size and live body coloration of opah (Teleostei: Lampridae: Lampris species)? Ichthyol. Res. 2012, 59, 272–275. [Google Scholar] [CrossRef]
- Runcie, R.M.; Dewar, H.; Hawn, D.R.; Frank, L.R.; Dickson, K.A. Evidence for cranial endothermy in the opah (Lampris guttatus). J. Exp. Biol. 2009, 212, 461–470. [Google Scholar] [CrossRef] [Green Version]
- Francis, M.; Griggs, L.; Maolagain, O.C. Growth rate, age at maturity, longevity and natural mortality rate of moonfish (Lampris guttatus). In Final Research Report for Ministry of Fisheries Research Project TUN2003-01; National Institute of Water and Atmospheric Research: Aukland, New Zeland, 2004; p. 28. [Google Scholar]
- Jackson, G.D.; Buxton, N.G.; George, M.J.A. Diet of the southern opah Lampris immaculatus on the Patagonian Shelf; the significance of the squid Moroteuthis ingens and anthropogenic plastic. Mar. Ecol. Prog. Ser. 2000, 206, 261–271. [Google Scholar] [CrossRef] [Green Version]
- Scales, K.L.; Hazen, E.L.; Jacox, M.G.; Castruccio, F.; Maxwell, S.M.; Lewison, R.L.; Bograd, S.J. Fisheries bycatch risk to marine megafauna is intensified in Lagrangian coherent structures. Proc. Natl. Acad. Sci. USA 2018, 115, 7362–7367. [Google Scholar] [CrossRef] [PubMed]
- Wegner, N.C.; Snodgrass, O.E.; Dewar, H.; Hyde, J.R. Whole-body endothermy in a mesopelagic fish, the opah, Lampris guttatus. Science 2015, 348, 786–789. [Google Scholar] [CrossRef] [PubMed]
- Bo, J.; Lv, W.Q.; Sun, N.; Wang, C.; Wang, K.; Liu, P.; Feng, C.G.; He, S.P.; Yang, L.D. Opah (Lampris megalopsis) genome sheds light on the evolution of aquatic endothermy. Zool. Res. 2022, 43, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Spinola, M. Lettre sur quelques poissons peu connus du gulfe de Gênes. Ann. du Muséum d’Histoire Nat. 1807, 10, 366–380. [Google Scholar]
- Risso, A. Histoire Naturelle Des Principales Productions de l’Europe Méridionale et Particulièrement de Celles des Environs de Nice et des Alpes Maritimes; Chez, F.-G., Ed.; Levrault, Libraire: Paris, France, 1826. [Google Scholar]
- Parin, N.; Kukuyev, N. Reestablishment of the Validity of Lampris immaculatus Gilchrist 1905, and the Geographical Distribution of Lampridae. J. Ichthyol. 1983, 23, 1–12. [Google Scholar]
- Ariola, V. Pesci nuovi o rari per il Golfo di Genova. Ann. Mus. Civ. Stor. Nat. Genova 1904, 41, 153–168. [Google Scholar]
- Cuvier, G.; Cuvier, G. Valenciennes Histoire Naturelle Des Poissons; Chez, F.G., Ed.; Levrault: Paris, France, 1828. [Google Scholar]
- Cattaneo-Vietti, R.; Bava, S. La tonnarella e la pesca tradizionale a Camogli. In Le Mani Recco; Le Mani: Recco, Italy, 2009. (In Italian) [Google Scholar]
- Tortonese, E. Fauna d’Italia: Osteichthyes (Pesci Ossei); Edizioni Calderini; Parte Seco.; Calderini: Bologna, Italy, 1970. [Google Scholar]
- Andaloro, F.; Di Natale, A. Ritrovamento di un esemplare di Lampris guttatus (briinmich, 1788) (pisces, lampridae) nelle acque del basso tirreno. Mem. Biol. Mar. Oceanogr. 1979, 9, 111–113. [Google Scholar]
- Psomadakis, P.N.; Scacco, U.; Vacchi, M. Recent findings of some uncommon fishes from the central Tyrrhenian Sea. Cybium 2006, 30, 297–304. [Google Scholar]
- Bego, F.; Kashta, L. First record of opah (Lampris guttatus brünnich, 1788) in albanian marine waters (brief communication). Albanian J. Nat. Tech. Sci. 2012, 32, 143–148. [Google Scholar]
- Sprem, J.S.; Dobroslavic, T.; Kozul, V.; Prusıina, I.; Onofri, V.; Antolovıć, N. New record of Lophotus lacepede (Giorna, 1809) and Lampris guttatus (Brünnich, 1788) in the southeastern Adriatic Sea (Croatian coast). Cah. Biol. Mar. 2014, 55, 371–373. [Google Scholar]
- Dulcic, J.; Jardas, I.; Pallaoro, A. New record of opah Lampris guttatus (Lampridae) in the Adriatic waters with a review of Adriatic records. Cybium 2005, 29, 195–197. [Google Scholar]
- Katurić, M. Lampris luna Lin 6. Rad Jugosl. Akad. Znan. Umjet. 1902, 149, 89–92. [Google Scholar]
- Crnković, D. Tunere i njihove neobične lovine. [Tunafishing stations and their unusual catches.]. Morsko Ribar. 1957, 1, 23–24. [Google Scholar]
- Sinis, A.I. First documented record of Lampris guttatus (Brünnich, 1788) opah, in Greek seas. J. Biol. Res. 2004, 2, 101–104. [Google Scholar]
- Corsini-Foka, M. Uncommon fishes from Rhodes and nearby marine region (SE Aegean Sea, Greece). J. Biol. Res. 2009, 12, 125–133. [Google Scholar]
- Ergüden, D.; Ayas, D.; Altun, A.; Alag, S.; Bayhan, Y.K. First Record of Lampris guttatus (Brünnich, 1788) in North-Eastern Mediterranean (Mersin Bay, Turkey). Available online: https://www.semanticscholar.org/paper/First-Record-of-Lampris-guttatus-(Brünnich%2C-1788)-Erg#xFC;den-Ayas/f8557cdea4362c08e3d62ec3b69feb59823c2316 (accessed on 8 May 2022).
- Ennajar, S.; Saidi, B.; Bradai, M.N. The first record of Lampris guttatus (brünnich, 1788) in the tunisian coasts (central mediterranean sea). Bull. L’institut Natl. Des Sci. Technol. La Merde Salammbô 2020, 47, 189–193. [Google Scholar]
- Francour, P.; Cottalorda, J.-M.; Aubert, M.; Bava, S.; Colombey, M.; Gilles, P.; Kara, H.; Lelong, P.; Mangialajo, L.; Miniconi, R. Recent occurrences of opah, Lampris guttatus (actinopterygii, lampriformes, lampridae), in the western mediterranean sea. Acta Ichthyol. Piscat. 2010, 40, 91–98. [Google Scholar] [CrossRef] [Green Version]
- Fischer, W. Fiches FAO d’identification des especes pour les besoins de la peche.(Rev 1). Mediterranee et mer Noire. Zone de Peche 37. Vertebres 1987, 2. [Google Scholar]
- Koeda, K.; Ho, H.-C. A new record of the genus Lophotus (Lampridiformes: Lophotidae) in Taiwan. Platax 2017, 2017, 55–61. [Google Scholar]
- Quignard, J.P.; Tomasini, J.A. Mediterranean fish biodiversity. Biol. Mar. Mediterr. 2000, 7, 1–66. [Google Scholar]
- Senou, H.; Kobayashi, Y.; Kobayashi, N. Coastal fishes of the Miyako group, the Ryukyu islands, Japan. Bull. Kanagawa Prefect. Mus. Nat. Sci. 2007, 36, 47–74. [Google Scholar]
- Shinohara, G.; Shirai, S.M.; Nazarkin, M.V.; Yabe, M. Preliminary list of the deep-sea fishes of the Sea of Japan. Bull. Natl. Mus. Nat. Sci. Ser. A 2011, 37, 35–62. [Google Scholar]
- Craig, M.T.; Hastings, P.A.; Pondella, D.J. Notes on the systematics of the crestfish genus Lophotus (Lampridiformes: Lophotidae), with a new record from California. Bull. Calif. Acad. Sci. 2004, 103, 57–65. [Google Scholar]
- Soljan, T. Fishes of the Adriatic (Ribe Jadrana).Fauna Et Flora Adriatica, Vol. 1, Pisces; Belgrad Nolit: Belgrad, Serbia, 1948. [Google Scholar]
- Kolombatović, G. Notizie ittiologiche; 1890. [Google Scholar]
- Morović, D. The contribution to the Adriatic fisheries. Poseb. Izd. Instituta za Oceanogr. i Ribar. Split 1950, 1, 106–107. [Google Scholar]
- Palmer, G. Fishes of the North-Eastern Atlantic and Mediterranean; Whitehead, P.J.P., Bauchot, M.-L., Hureau, J.-C., Nielsen, J., Tortonese, E., Eds.; UNESCO: Paris, France, 1986; Volume 1. [Google Scholar]
- Bauchot, M.L. Poissons osseux. Fiches FAO D’identification Pour Les Besoins La Pêche.(Rev. 1). Méditerranée Mer Noire. Zone Pêche 1987, 37, 891–1421. [Google Scholar]
- Papaconstantinou, C. Fauna Graeciae, IV Pisces. Check-List Mar. Fishes Greece. Athens Greece Natl. Cent. Mar. Res. Hell. Zool. Soc. Athens 1988. [Google Scholar]
- Dulčić, J.; Ahnelt, H. How many specimens of the crested oarfish, Lophotus lacepede Giorna, 1809 (Pisces: Lophotidae), were caught in the Adriatic Sea? Acta Adriat. Int. J. Mar. Sci. 2007, 48, 39–43. [Google Scholar]
- Dulčić, J.; Soldo, A. New finding of crested oarfish Lophotus lacepede (Lophotidae), in the Adriatic Sea. Cybium 2008, 32, 93–99. [Google Scholar]
- Magazzù, G.; Zaccone, G. Su di un altro esemplare di Lophotus cepedianus (Giorna) catturato nelle acque di capo Peloro, Messina. Mem. di Biol. Mar. e di Oceanogr. 1971, 53–57. [Google Scholar]
- Giuffre, G.; Gugliotta, R.; Nicotra, S. On a specimen of Lophotus lacepedei Giorna 1809 (Pisces: Lophotidae), stranded on the Sicilian coast of the Strait of Messina. Mem. di Biol. Mar. e di Oceanogr. 1980, 10, 9–18. [Google Scholar]
- Psomadakis, P.N.; Giustino, S.; Vacchi, M. Mediterranean fish biodiversity: An updated inventory with focus on the Ligurian and Tyrrhenian seas. Zootaxa 2012, 3263, 1–46. [Google Scholar] [CrossRef]
- Tripepi, S.; Fera, D.; Sperone, E. New finding of crested oarfish Lophotus lacepedei Giorna, 1809 (Lampridiformes, Lophotidae) in Southern Italy. J. Ichthyol. 2004, 44, S150. [Google Scholar]
- Bussotti, S.; Guidetti, P.; Terlizzi, A. Stranding of Lophotes lacepedei (Giorna, 1809) and a young specimen of Trachypterus trachypterus (Gmelin, 1789) at Ischia Island (Gulf of Naples, western Mediterranean). Doriana 1999, 322, 1–5. [Google Scholar]
- Portas, F.; Del Cerro, L. Lophotus lacepedei Giorna, 1809 (Pisces, Lophotidae): Primera cita para las costas españolas. Miscel Lània Zoològica 1979, 5, 188–189. [Google Scholar]
- Rey, J.C. Captura de un ejemplar de Lophotus lacepedei Giorna, 1809 (Pisces, Lophotidae) en el estrecho de Gibraltar. Memórias do Mus. do Mar-Série Zoológica 1983, 3, 1–16. [Google Scholar]
- Rodriguez, J.M.; Alvarez, I.; López-Jurado, J.L.; Garcia, A.; Balbín, R.; Alvarez-Berastegui, D.; Torres, A.P.; Alemany, F. Environmental forcing and the larval fish community associated to the Atlantic bluefin tuna spawning habitat of the Balearic region (Western Mediterranean), in early summer 2005. Deep Sea Res. Part I Oceanogr. Res. Pap. 2013, 77, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Bilecenoğlu, M.; Murat, K.; Irmak, E. A new mesopelagic fish for Turkish seas, Lophotus lacepede Giorna, 1809 (Pisces: Lophotidae). Ege J. Fish. Aquat. Sci. 2001, 18, 537–539. [Google Scholar]
- Dalyan, C.; Gönülal, O.; Kesici, N.B.; Eryilmaz, L.; Tunçer, S.; Öztekin, A. New and confirmed records and rare occurrences of some deep sea fishes in the Turkish waters of the northern Aegean Sea (Mediterranean Sea). FishTaxa 2021, 21, 1–18. [Google Scholar]
- Aga-Spyridopoulou, R.N.; Giovos, I.; Kleitou, P.; Christidis, A.; Langeneck, J.; Kalogirou, S. Preliminary results on the distribution extension of five data-limited fish species in the eastern Mediterranean Sea. In Proceedings of the 14th ICZEGAR Conference Thessaloniki, Thessaloniki, Greece, 27–30 June 2019; pp. 27–30. [Google Scholar]
- Yapici, S. New and additional records of rare fish species from the Anatolian coasts of Turkey. Mugla J. Sci. Technol. 2019, 5, 13–16. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.; Hammoud, V.; Fandi, O.; Capapé, C. First substantiated record of crested oarfish Lophotus lacepede (osteichthyes: Lophotidae) from the Syrian coast (eastern Mediterranean Sea). Ann. Ser. Hist. Nat. 2021, 31, 205–210. [Google Scholar] [CrossRef]
- Osório, B. Nota sôbre algumas espécies de peixes que vivem no Atlântico ocidental. Arq. Da Univ. Lisboa 1917, 4, 103–131. [Google Scholar]
- Heemstra, P.C.; Kannmeyer, S.X. The families Trachipertidae and Radiicephalidae (Pisces, Lampriformes) and a new species of Zu from South Africa. Ann. S. Afr. Mus. 1984, 94, 13–39. [Google Scholar]
- Moser, H.G. Lampridiformes, Lophotidae, Radiicephalidae, Trachipteridae. In The Early Stages of Fishes in the California Current Region; California Cooperative Oceanic Fisheries Investigations Atlas; US Department of the Interior, Minerals Management Service: Washington, DC, USA, 1996; pp. 659–677. [Google Scholar]
- Harrison, C.M.H.; Palmer, G. On the Neotype of Radiicephalus Elongatus Osorio with Remarks on Its Biology; British Museum: London, UK, 1968; Volume 16. [Google Scholar]
- Stocco, L.B.; Joyeux, J.-C. Distribution of fish larvae on the Vitória-Trindade Chain, southwestern Atlantic. Check List 2015, 11, 1590. [Google Scholar] [CrossRef] [Green Version]
- Karrer, C. Über drei mesopelagische fischarten aus dem Golf von Guinea. Mitteilungen aus dem Museum für Naturkd. Berlin. Zool. Museum Und Inst. Für Spez. Zool. 1976, 52, 177–182. [Google Scholar] [CrossRef]
- Bolshakova, Y.; Evseenko, S.A. On species composition of ichthyoplankton of the Mid-Atlantic Ridge (South Atlantic). J. Ichthyol. 2016, 56, 522–533. [Google Scholar] [CrossRef]
- Butler, J.L. Fishes Collected by Midwater Trawls during Two Cruises of the David Starr Jordan in the Northeastern Pacific Ocean, April-June and September-October, 1972; Southwest Fisheries Science Center: La Jolla, CA, USA, 1997; Volume 244. [Google Scholar]
- Gomon, M.F.; Bray, D.J.; Kuiter, R.H. Fishes of Australia’s Southern Coast; New Holland Chatswood: Wahroonga, Australia, 2008; ISBN 1877069183. [Google Scholar]
- Loeb, V.J. Larval fishes in the zooplankton community of the North Pacific Central Gyre. Mar. Biol. 1979, 53, 173–191. [Google Scholar] [CrossRef]
- Robins, C.R.; Ray, G.C.; Douglass, J. A Field Guide to Atlantic Coast Fishes of North America; Houghton Mifflin Harcourt: Boston, MA, USA, 1986; ISBN 0395975158. [Google Scholar]
- Chakrabarty, P. Systematics, Biology, and Distribution of the Species of the Oceanic Oarfish Genus Regalecus (Teleostei, Lampridiformes, Regalecidae)Systematics, Biology, and Distribution of the Species of the Oceanic Oarfish Genus Regalecus (Teleostei, Lampridiformes, R; Publications Scientifiques du Muséum Paris: Paris, France, 2013; Volume 2013, ISBN 2856536778. [Google Scholar]
- Forsgren, K.L.; Jamal, H.; Barrios, A.; Paig-Tran, E.W.M. Reproductive Morphology of Oarfish (Regalecus russellii). Anat. Rec. 2017, 300, 1695–1704. [Google Scholar] [CrossRef] [Green Version]
- Oka, S.; Nakamura, M.; Nozu, R.; Miyamoto, K. First observation of larval oarfish, Regalecus russelii, from fertilized eggs through hatching, following artificial insemination in captivity. Zool. Lett. 2020, 6, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Peng, X.; Yang, C.-M.; Chen, X.; Chen, S.; Qin, S. Complete mitochondrial genome and the phylogenetic position of the giant oarfish (Regalecus glesne). Mitochondrial DNA Part B 2019, 4, 2125–2126. [Google Scholar] [CrossRef] [Green Version]
- Eschmeyer, W.N.; Herald, E.S. A Field Guide to Pacific Coast Fishes; Houghton Mifflin Harcourt: Boston, MA, USA, 1983; ISBN 061800212X. [Google Scholar]
- Roberts, T.R. Payanak as a mythical animal and as the living species Regalecus glesne (Oarfish, Regalecidae, Lampridiformes). Nat. Hist. Bull. Siam. Soc. 2002, 50, 211–224. [Google Scholar]
- Ruiz, A.E.; Gosztonyi, A.E. Records of regalecid fishes in Argentine waters. Zootaxa 2010, 2509, 62–66. [Google Scholar] [CrossRef] [Green Version]
- Jurado, V. Presencia de Regalecus glesne, Ascanius 1772 (Rey de los arenques, pez remo) En aguas ecuatorianas. Rev. Ciencias Del. Mar. Y Limnol. 2010, 4, 115–118. [Google Scholar]
- Schmitter-Soto, J.J. The oarfish, Regalecus glesne (Teleostei: Regalecidae), in the western Caribbean. Caribb. J. Sci. 2008, 44, 125–128. [Google Scholar] [CrossRef]
- Benfield, M.C.; Cook, S.; Sharuga, S.; Valentine, M.M. Five in situ observations of live oarfish Regalecus glesne (Regalecidae) by remotely operated vehicles in the oceanic waters of the northern Gulf of Mexico. J. Fish Biol. 2013, 83, 28–38. [Google Scholar] [CrossRef]
- Vayssiere, M.A. Note zoologique et anatomique sur un Regalecus (Gymnetrus) gladius Cuv. et Valen. Pris dans le golfe de Marseille. Bull. Mus. Nat. Hist. Natur. 1917, 23, 15–25. [Google Scholar]
- Giglioli, E.H. Elenco dei Mammiferi, Degli Uccelli e Dei Rettili Ittiofagi Appartenenti Alla Fauna Italica, e Catalogo Degli Anfibi e dei Pesci Italiani; Stamperia Reale: Rome, Italy, 1880. [Google Scholar]
- Vinciguerra, D. Intorno ai Regalecus del golfo di Genova e di altre località italiane. Ann. Del Mus. Civ. Di Stor. Nat. Di Genova 1918, 48, 76–92. [Google Scholar]
- Damiani, G. Di un “Regalecus gladius” Walb. all’isola d’elba, con note sui“Regalecus” mediterranei. Boll. Della Soc. Zool. Ital. 1913, 1, 329–339. [Google Scholar]
- Guiglia, D. Nuova cattura di Regalecus glesne asc. nel golfo di genova. Ann. Del Mus. Civ. Di Stor. Nat. Di Genova 1950, 64, 288–290. [Google Scholar]
- Psomadakis, P.N.; Bottaro, M.; Doria, G.; Garibaldi, F.; Giustino, S.; Vacchi, M. Notes on the Regalecus glesne occurring in the Gulf of Genova and in Liguro-Provencal waters (NW Mediterranean)(Pisces, Lampridiformes, Regalecidae). Ann. Del Mus. Civ. Di Stor. Nat. G. Doria 2008, 99, 549–571. [Google Scholar]
- Quero, J.-C.; Spitz, J.; Vayne, J.-J. Observations ichtyologiques effectuées en 2002. In Proceedings of the Annales de la Société des sciences Naturelles de la Charente-Maritime; Société des Sciences Naturelles de la Charente-Maritime: La Rochelle, France, 2003; Volume 9, pp. 275–279. [Google Scholar]
- Lozano-Cabo, F. Nota sobre el hallazgo en Mazarrôn de una especie de pez Regalecus glesne (Ascanius), poco comûn en el Méditerranée. Boletín La Real Soc. Española Hist. Nat. Sección Biológica 1969, 67, 29–31. [Google Scholar]
- Berdar, A.; Guglielmo, L.; Giacobbe, S. Ritrovamento di tre giovani esemplari di Regalecus glene Ascanius, 1772 spiaggiati ad Oliveri (Messina). Atti Soc. Peloritana 1975, 21, 123–131. [Google Scholar]
- Cavallaro, G.; Cavaliere, A.; Berdar, A. [Regalecus glesne Ascanius (Pisces: Regalecidae) washed up on the beach of the straits of Messina [Italy]].[Italian]. Mem. Di Biol. Mar. Di Oceanogr. 1980, 10, 135–137. [Google Scholar]
- Padovani, C. Prima cattura di Regalecus gladius (Walb.) nel mare adriatico. Bollettini di Pesca, Pisc. Iscultura e Idrobiol. 1933, 11, 102–107. [Google Scholar]
- Dulčić, J.; Dragičević, B.; TuTman, P. Record of Regalecus glesne (Regalecidae) from the eastern Adriatic Sea. Cybium 2009, 33, 339–340. [Google Scholar]
- Ondrias, J. A list of the fresh and sea water fishes of Greece, Prakt. Inst. Ocean. Fish Res. 1971, 10, 23. [Google Scholar]
- Papacostantinou, C. Fauna Greciae; National Centre for Marine Research: Athens, Greece, 1988; Volume 4. [Google Scholar]
- Nishimura, S. Additional information on the biology of the dealfish Trachipterus ishikawai Jordan & Snyder. Bull. Jpn. Sea Reg. Fish. Res. Lab. 1964, 13, 127–129. [Google Scholar]
- Fitch, J.E. The Ribbonfishes (Family Trachipteridae) of the Eastern Pacific Ocean, with a Description of a New Species. Calif. Fish Game 1964, 50, 228–240. [Google Scholar]
- Lipej, L.; Trkov, D.; Mavrič, B. Occurrence of ribbon fish (Trachipterus trachypterus) in Slovenian waters (northern Adriatic Sea). In Proceedings of the Annales: Series Historia Naturalis; Scientific and Research Center of the Republic of Slovenia: Ljubjana, Slovenia, 2018; Volume 28, pp. 129–134. [Google Scholar]
- Figueiredo, I.; Moura, T.; Gordo, L.S. Vertebrae counting—A way to resolve species identification of the genus Trachipterus (Osteichthyes:Trachipteridae). Mar. Biodivers. Rec. 2008, 1. [Google Scholar] [CrossRef]
- Okiyama, M. An atlas of the early stage fishes in Japan. Atlas Early Stage Fishes Jpn. 1988, 312–317. [Google Scholar]
- Savinykh, V.F.; Baitalyuk, A.A. Taxonomic status of ribbonfishes of the genus Trachypterus (Trachipteridae) from the northern part of the Pacific Ocean. J. Ichthyol. 2011, 51, 581–589. [Google Scholar] [CrossRef]
- Richards, W.J. Early Stages of Atlantic Fishes: An Identification Guide for the Western Central North Atlantic; CRC Press: Boca Raton, FL, USA, 2006; Volume 43, ISBN 0429210329. [Google Scholar]
- Olney, J.E.; Naplin, A. Eggs of the Scalloped Ribbonfish, Zu cristatus, (Pisces: Trachipteridae) in the Western North Atlantic. Copeia 1980, 1980, 165. [Google Scholar] [CrossRef]
- Ogilby, J.D. On a Trachypterus from New South Wales. In Proceedings of the Linnean Society of New South Wales; BioStor: Wexford, Ireland, 1898; Volume 22, pp. 646–659. [Google Scholar]
- Rathnasuriya, M.I.G.; Mateos-Rivera, A.; Bandara, A.G.G.C.; Skern-Mauritzen, R.; Jayasinghe, R.P.P.K.; Krakstad, J.O.; Dalpadado, P. DNA barcoding confirms the first record of a Desmodema polystictum (Ogilby, 1898) egg and all-time high adult catches in the Indian Ocean. Mar. Biodivers. Rec. 2019, 12, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Shirke, S.S.; Ramachandran, S.; Pradeep, H.D.; Musaliyarakam, N.; Devi, S.M.; Sinha, M.K. First record of the Taper-tail ribbonfish Zu elongatus Heemstra & Kannemeyer, 1984 from Indian EEZ. FishTaxa 2017, 2, 43–47. [Google Scholar]
- Palmer, G. The Dealfishes (Trachipteridae) of the Mediterranean and north east Atlantic. Bull. Br. Museum Nat. Hist. Zool. 1961, 7, 335–351. [Google Scholar] [CrossRef]
- Rosenblatt, R.H.; Butler, J.L. The ribbonfish genus Desmodema, with the description of a new species (Pisces, Trachipteridae). Fish. Bull. 1977, 75, 843–855. [Google Scholar]
- Costa, F. Atlante Dei Pesci Dei Mari Italiani; Gruppo Ugo Mursia Editore: Milano, Italy, 1991; ISBN 9788842522591. [Google Scholar]
- Jardas, I. Contribution à la connaissance des Trachiptères dans la mer Adriatique. 1. Trachipterus trachypterus (Gmelin, 1789). Acta Adriat. 1980, 21, 3–17. [Google Scholar]
- Marcuzzi, G. Le collezioni dellex Istituto di biologia marina di Rovigno conservate presso la Stazione Idrobiologica di Chioggia. Soc. Tipogr. 1972, 84, 219. [Google Scholar]
- Bussani, M. Ricomparsa nel Golfo di Trieste di alcune specie ittiche. Hydrores Inf. 1992, 9, 5–7. [Google Scholar]
- Dulcic, J. First record of ribbon fish, Trachipterus trachypterus, from the eastern Adriatic. Cybium 1996, 20, 101–102. [Google Scholar]
- Jardas, I.; Pallaoro, A. The record of Sphoeroides cutaneus (Gunther, 1870)(Pisces, Tetraodontidae) in the Adriatic Sea. Oebalia 1996, 22, 83–90. [Google Scholar]
- Tortonese, E.; Trotti, L. Catalogo dei pesci del Mare Ligure. Atti. Accad. Ligure Sci. Lett. 1949, 6, 1–118. [Google Scholar]
- Cau, A. Secondo contributo alla conoscenza dell’ ittiofauna batiale dei mari circostanti la Sardegna meridionale (Osteichthyes). Quad. Civ. Staz. Idrobiol. Milano 1980, 8, 39–43. [Google Scholar]
- Tiralongo, F.; Crocetta, F.; Riginella, E.; Lillo, A.O.; Tondo, E.; Macali, A.; Mancini, E.; Russo, F.; Coco, S.; Paolillo, G.; et al. Snapshot of rare, exotic and overlooked fish species in the Italian seas: A citizen science survey. J. Sea Res. 2020, 164, 101930. [Google Scholar] [CrossRef]
- Mytilineou, C.; Anastasopoulou, A.; Christides, G.; Bekas, P.; Smith, C.J.; Papadopoulou, K.N.; Lefkaditou, E.; Kavadas, S. New records of rare deep-water fish species in the Eastern Ionian Sea (Mediterranean Sea). J. Nat. Hist. 2013, 47, 1645–1662. [Google Scholar] [CrossRef]
- Golani, D. The marine ichthyofauna of the Eastern Levant—History, inventory, and characterization. Isr. J. Ecol. Evol. 1996, 42, 15–55. [Google Scholar]
- Gökoğlu, M.; Özen, M.R. First Record of Trachipterus trachypterus (Gmelin, 1789) in the Gulf of Antalya (Turkey). Acta Aquat. Turc. 2021, 17, 505–507. [Google Scholar] [CrossRef]
- Kaminas, A.; Minasidis, V.; Doumpas, N.; Naasan Aga Spyridopoulou, R.T.F. Occurrence of Trachipterus trachypterus and Zu cristatus in the Greek Seas: Contribution of citizen science projects in rare species monitoring. In Proceedings of the HydroMedit 2021; University of Thessaly: Thessaly, Greece, 2021. [Google Scholar]
- Bañón, R.; Tejerina, R.; Morales, X.; Alonso-Fernández, A.; Barros-García, D.; Carlos, A. De Unusual occurrences of fishes along the Northeast Atlantic: New biological and distributional data. Mediterr. Mar. Sci. 2019, 20, 189–196. [Google Scholar] [CrossRef] [Green Version]
- Mohan, S.; Selvanidhi, S.; Rajapackiam, S. First Record of Scalloped Ribbonfish Zu cristatus (Bonelli, 1819) at Chennai; Central Marine Fisheries Research Institute: Cochin, India, 2011. [Google Scholar]
- Oliver, M. Cita de peces no frecuentes pescados en aguas de Mallorca. Bolletí La Soc. d’Història Nat. Les Balear. 1955, 1, 45–48. [Google Scholar]
- Ibáñez, M.; Gállego, L. A New Record of a Zu Cristatus (Trachipteridae Pisces) Off the Coast of Blanes (Spain). Vie Milieu 1974, 523–526. [Google Scholar]
- Roig, A.; Zoològica, M.; i Alted, M.D. Nota sobre la captura de dos Zu cristatus (Bonelli, 1820) en aguas del litoral catalán (Pisces, Trachipterigidae). Miscel·Lània Zoològica 1980, 6, 152–154. [Google Scholar]
- Bonelli, F.A. Description d’une nouvelle espèce de poisson de la Méditerranée appartenant au genre Trachyptère avec des observations sur les caractères de ce même genre. Mem. Della R. Accad. Delle Sci. Di Torino 1820, 24, 485–494. [Google Scholar]
- Tortonese, E. Cattura di Trachypterus cristatus Bon. e note sui Trachypteridae del Mar Ligure. Doriana 1958, 2, 1–5. [Google Scholar]
- Bianco, P.G.; Zupo, V.; Ketmaier, V. Occurrence of the scalloped ribbonfish Zu cristatus (Lampridiformes) in coastal waters of the central Tyrrhenian Sea, Italy. J. Fish Biol. 2006, 68, 150–155. [Google Scholar] [CrossRef]
- Bradai, M.N.; El Ouaer, A. New record of the scalloped ribbon fish, Zu cristatus (Osteichthyes: Trachipteridae) in Tunisian waters (central Mediterranean). ANZIAM J. 2014, 5, e59. [Google Scholar] [CrossRef]
- Postel, E. Capture d’un trachyptère<Trachypterus cristatus> Bonelli en Baie de Tunis. Bulletin; Station Océanographique de Salammbô: Carthage, Tunis, 1955; Volume 51, pp. 69–71. [Google Scholar]
- Dulcic, J.; Dragicevic, B.; Pavicic, M.; Ikica, Z.; Joksimovic, A.; Markovic, O. Additional records of non-indigenous, rare and less known fishes in the eastern adriatic/Nuove segnalazioni di pesci non indigeni, rari e meno conosciuti nell’Adriatico orientale. In Proceedings of the Annales: Series Historia Naturalis; Scientific and Research Center of the Republic of Slovenia: Ljubjana, Slovenia, 2014; Volume 24, p. 17. [Google Scholar]
- Dunne, J.A.; Maschner, H.; Betts, M.W.; Huntly, N.; Russell, R.; Williams, R.J.; Wood, S.A. The roles and impacts of human hunter-gatherers in North Pacific marine food webs. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef]
- Fitch, J.E.; Lavenberg, R.J. Deep-Water Teleostean Fishes of California; University of California Press: Berkeley, CA, USA, 1970; Volume 56. [Google Scholar]
- Heemstra, P.C. Family No. 118: Veliferidae. Smith’s Sea Fishes Eds. Smith MM Heemstra PC Macmillan Johannesbg. S. Afr. 1986, 398–399. [Google Scholar]
- Walters, V. Synopsis of the Lampridiform Suborder Veliferoidei. Copeia 1960, 1960, 245. [Google Scholar] [CrossRef]
- Al-Mamry, J.M.; Jawad, L.A. Sailfin velifer, Velifer hypselopterus Bleeker, 1879 (Perciformes, Veliferidae) in Oman waters: A new substantiated record from the Arabian Sea coast of Oman. Thalass. Salentina 2021, 43, 29–34. [Google Scholar]
- May, J.L.; Maxwell, J.G.H. Trawl fish from temperate waters of Australia. CSRio. Div. Fishes Res. Tasmania. 1986, 492. [Google Scholar]
- Stephenson, A.B. Metavelifer multiradiatus (Regan, 1907)(Pisces: Veliferidae), a new record from New Zealand waters. Rec. Auckl. Inst. Museum 1977, 143–144. [Google Scholar]
- Uyeno, T.E.F.; Masuda, H.; Amaoka, K.; Araga, C.; Uyeno, T.; Yoshino, T. The Fishes of the Japanese Archipelago. Tokai Univ. 1984, 190–191. [Google Scholar]
- Russell, B.C.; Houston, W. Offshore fishes of the Arafura Sea. Beagle Rec. Museums Art Gall. North. Territ. 1989, 6, 69–84. [Google Scholar] [CrossRef]
- Jawad, L.A.; Jufaili, S.M. Al Confirmation of the record of four teleosts into the Persian Gulf coast of Oman. Cah. Biol. Mar. 2022, 63, 13–18. [Google Scholar] [CrossRef]
- IUCN. IUCN The IUCN Red List of Threatened Species; Version 2021-3; International Union for Conservation of Nature and Natural Resources: Gland, Switzerland, 2021. [Google Scholar]





| Family | Genus | Species |
|---|---|---|
| Lampridae (Gill 1862) | Lampris (Retzius, 1799) | Lampris australensis (Underkoffler, Luers, Hyde and Craig, 2018) |
| Lampris guttatus (Brünnich, 1788) | ||
| Lampris immaculatus (Gilchrist, 1904) | ||
| Lampris incognitus (Underkoffler, Luers, Hyde and Craig, 2018) | ||
| Lampris megalopsis (Underkoffler, Luers, Hyde and Craig, 2018) | ||
| Lophotidae (Bonaparte, 1845) | Eumecichthys (Regan, 1907) | Eumecichthys fiski (Günther, 1890) |
| Lophotus (Giorna, 1809) | Lophotus capellei (Temminck and Schlegel, 1845) | |
| Lophotus guntheri (Johnston, 1883), | ||
| Lophotus lacepede (Giorna, 1809), | ||
| Lophotus machadoi (Miranda Ribeiro, 1927) | ||
| Radiicephalidae (Osorio, 1917) | Radiicephalus (Osório, 1917) | Radiicephalus elongatus (Osório, 1917) |
| Radiicephalus kessinger (Koeda and Ho, 2018) | ||
| Regalecidae (Gill, 1884) | Agrostichthys (Phillipps, 1924) | Agrostichthys parkeri (Benham, 1904) |
| Regalecus (Ascanius, 1772) | Regalecus glesne (Ascanius, 1772) | |
| Regalecus russellii (Cuvier, 1816) | ||
| Trachipteridae (Swainson, 1839) | Desmodema (Walters and Fitch, 1960) | Desmodema lorum (Rosenblatt and Butler, 1977) |
| Desmodema polystictum (Ogilby, 1898) | ||
| Trachipterus (Goüan, 1770) | Trachipterus altivelis (Kner, 1859) | |
| Trachipterus arcticus (Brünnich, 1788) | ||
| Trachipterus fukuzakii (Fitch, 1964) | ||
| Trachipterus ishikawae (Jordan and Snyder, 1901) | ||
| Trachipterus jacksonensis (Ramsay, 1881) | ||
| Trachipterus trachypterus (Gmelin, 1789) | ||
| Zu (Walters and Fitch, 1960) | Zu cristatus (Bonelli, 1819) | |
| Zu elongatus (Heemstra and Kannemeyer, 1984) | ||
| Veliferidae (Bleeker, 1859) | Metavelifer (Walters, 1960) | Metavelifer multiradiatus (Regan, 1907) |
| Velifer (Temminck and Schlegel, 1850) | Velifer hypselopterus (Bleeker, 1879) |
| Family, Genus | Species | Year | Mediterranean Area | Number of Specimens | References |
|---|---|---|---|---|---|
| Lampridae, Lampris | Lampris guttatus | before 1807 | Ligurian Sea (Italy) | 1 | Spinola (1807) |
| before 1826 | Nice (France) | 1 | Risso (1826) | ||
| 1829 | Toulon (France) | 1 | Cuvier and Valenciennes (1835) | ||
| 1829 | Marseille (France) | 1 | Cuvier and Valenciennes (1835) | ||
| 1898 | Viareggio (Italy) | 1 | Ariola (1904) | ||
| 1901 | Camogli (Italy) | 1 | Ariola (1904) | ||
| 1902 | Novigrad Sea (Croatia) | 2 | Katurić (1902) | ||
| 1956 | Bakar Bay (Croatia) | 1 | Crnković (1957) | ||
| before 1970 | Finale Ligure (Italy) | 1 | Tortonese (1970) | ||
| 1974 | Camogli (Italy) | 1 | Cattaneo and Bava (2009) | ||
| 1979 | Pizzo (Italy) | 1 | Andaloro and Di Natale (1979) | ||
| 1983 | Unknown | 1 | Parin and Kukuyew (1983) | ||
| 1994 | Neretva estuary (Croatia) | 1 | Bartulović (in Dulčić et al. 2005) | ||
| 1997 | Toulon/Embiez (France) | 1 | Francour et al. (2010) | ||
| 1997 | Bandol (France) | 1 | Francour et al. (2010) | ||
| 1998 | Anzio (Italy) | 3 | Psomadakis et al. (2006) | ||
| 2000 | Porquerolles Island (France) | 1 | Francour et al. (2010) | ||
| 2000 | Sète (France) | 1 | Francour et al. (2010) | ||
| 2001 | Anzio (Italy) | 1 | Psomadakis et al. (2006) | ||
| 2002 | Nea Skioni (Greece) | 1 | Sinis (2004) | ||
| 2003 | Embiez (France) | 1 | Francour et al. (2010) | ||
| 2003 | Vir Island (Croatia) | 1 | Dulčić et al. (2005) | ||
| 2004 | Bormes-les-Mimosas (France) | 2 | Francour et al. (2010) | ||
| 2007 | Antibes (France) | 2 | Francour et al. (2010) | ||
| 2007 | Embiez (France) | 1 | Francour et al. (2010) | ||
| 2007 | Marseille (France) | 1 | Francour et al. (2010) | ||
| 2007 | North Bastia (France) | 1 | Francour et al. (2010) | ||
| 2008 | Antibes (France) | 1 | Francour et al. (2010) | ||
| 2008 | Giens (France) | 1 | Francour et al. (2010) | ||
| 2008 | off Le Levant Island (France) | 1 | Francour et al. (2010) | ||
| 2008 | Karpathos Island (Greece) | 1 | Corsini-Foka (2009) | ||
| 2008 | off Nice (France) | 2 | Francour et al. (2010) | ||
| 2008 | Porquerolles Island (France) | 1 | Francour et al. (2010) | ||
| 2008 | off Cagnes sur Mer (France) | 1 | Francour et al. (2010) | ||
| 2008 | Saint-Raphaël (France) | 2 | Francour et al. (2010) | ||
| 2008 | Gouraya (Algeria) | 1 | Francour et al. (2010) | ||
| 2008 | Camogli (Italy) | 3 | Francour et al. (2010) | ||
| 2009 | Camogli (Italy) | 1 | Francour et al. (2010) | ||
| 2009 | Cannes (France) | 1 | Francour et al. (2010) | ||
| 2009 | East Corsica (France) | 1 | Francour et al. (2010) | ||
| 2009 | Radhima, Vlora Bay (Albania) | 1 | Bego and Kashta (2012) | ||
| 2012 | Mali Ston Bay (Croatia) | 1 | Šprem et al. (2014) | ||
| 2017 | off Erdemli coast (Turkey) | 1 | Ergüden et al. (2019) | ||
| 2021 | Ghar El Melh (Tunisia) | 1 | Ennajar et al. (2020) |
| Family, Genus | Species | Year | Mediterranean Area | Number of Specimens | References |
|---|---|---|---|---|---|
| Lophotidae, Lophotus | Lophotus lacepede | before 1890 | Adriatic Sea (Croatia) | 1 | Kolombatović (1890) |
| before 1948 | North Adriatic Sea | N.R. | Soljan (1948) | ||
| before 1950 | Adriatic Sea | 1 | Morović (1950) | ||
| 1970 | Strait of Messina (Italy) | 1 | Tortonese (1970) | ||
| before 1970 | Genova (Italy) | 1 | Tortonese (1970) | ||
| 1971 | Strait of Messina (Italy) | 1 | Magazzù and Zaccone (1971) | ||
| 1979 | Sitges (Spain) | 1 | Portas and Del Cerro (1979) | ||
| 1980 | Strait of Gibraltar | 1 | Ray (1983) | ||
| 1980 | Strait of Messina (Italy) | 1 | Giuffrè et al. (1980) | ||
| before 1986 | Ionian Sea | N.R. | Palmer (1986) | ||
| before 1987 | Central Mediterranean | N.R. | Bauchot (1987) | ||
| before 1988 | Greek Sea | N.R. | Papacostantinou (1988) | ||
| 1989 | Sitia, Crete Island (Greece) | 1 | Minos et al. (2015) | ||
| 1999 | Ischia Island (Italy) | 1 | Bussotti et al. (1999) | ||
| 2001 | Gökova Bay (Turkey) | 1 | Bilecenoglu et al. (2001) | ||
| 2002 | Calabria (Italy) | 1 | Tripepi et al. (2004) | ||
| 2003 | Souda Bay, Crete Island (Greece) | 1 | Minos et al. (2015) | ||
| 2005 | Balearic region (Spain) | 1 | Rodriguez et al. (2013) | ||
| 2007 | North Adriatic Sea (Croatia) | 2 | Dulčic and Ahnelt (2007) | ||
| 2008 | North Adriatic Sea (Croatia) | 1 | Dulčic and Soldo (2008) | ||
| 2011 | Southern Adriatic (Croatia) | 1 | Sprem et al. (2014) | ||
| 2011 | cape Poseidi (Greece) | 1 | Minos et al. (2015) | ||
| 2012 | Punta Licosa (Italy) | 1 | Psomadakis et al. (2012) | ||
| 2015 | San Vito Lo Capo (Italy) | 1 | Falsone et al. (2017) | ||
| 2016 | Aegean Sea (Greece) | 1 | Aga-Spyridopoulou et al. (2019) | ||
| 2017 | northern Aegean Sea (Turkey) | 1 | Dalyan et al. (2021) | ||
| 2017 | Aegean Sea (Greece) | 3 | Aga-Spyridopoulou et al. (2019) | ||
| 2018 | Aegean Sea (Greece) | 1 | Aga-Spyridopoulou et al. (2019) | ||
| 2018 | Çanakkale (Turkey) | 1 | Tunçer and Kanat (2019) | ||
| 2019 | Levantine Sea (Turkey) | 1 | Yapici (2019) | ||
| 2021 | Ammouliani Island (Greece) | 1 | Minasidis and Kaminas (2021) | ||
| 2021 | off Banias (Syria) | 1 | Ali et al. (2021) |
| Family, Genus | Species | Year | Mediterranean Area | Number of Specimens | References |
|---|---|---|---|---|---|
| Regalecidae, Regalecus | Regalecus glesne | 1826 | Nice (France) | 2 | Risso (1826) |
| 1830 | Nice (France) | 3 | Cuvier and Valenciennes (1835) | ||
| 1877 | Nice (France) | 1 | Giglioli (1880) | ||
| 1891 | Isola d’Elba (Italy) | 1 | Damiani (1918) | ||
| 1897 | Beaulieu saint-Jean (Francia) | 1 | Vayssiére (1917) | ||
| 1903 | Noli (Italy) | 1 | Ariola (1904) | ||
| 1906 | Borghetto S. Spirito (Italy) | 1 | Vinciguerra (1918) | ||
| 1908 | Arenzano (Italy) | 1 | Vinciguerra (1918) | ||
| 1910 | Monaco (France) | 1 | Vayssiére (1917) | ||
| 1913 | Castiglioncello (Italy) | 1 | Vinciguerra (1918) | ||
| 1915 | Albissola (Italy) | 1 | Vinciguerra (1918) | ||
| 1917 | S Margherita Ligure (Italy) | 1 | Vinciguerra (1918) | ||
| 1932 | Rimini (Italy) | 1 | Padovani (1933) | ||
| 1950 | Genova (Italy) | 1 | Guiglia (1950) | ||
| 1969 | Mazzarrón (Spain) | 1 | Lozano-Cabo (1969) | ||
| before 1970 | Ligurian Sea | N.R. | Tortonese (1970) | ||
| before 1971 | Aegean Sea | N.R. | Ondrias (1971) | ||
| 1974 | Olivieri (Italy) | 3 | Berdar et al. (1975) | ||
| 1980 | Strait of Messina (Italy) | 1 | Cavallaro et al. (1980) | ||
| before 1988 | Aegean Sea | N.R. | Papacostantinou (1988) | ||
| 1993 | Gennadi (Greece) | 1 | Corsini-Foka (2009) | ||
| 2002 | Marseille (France) | 2 | Quero et al. (2003) | ||
| 2003 | Arenzano (Italy) | 1 | Psomadakis et al. (2008) | ||
| 2009 | Stobrec (Croatia) | 1 | Dulčic et al. (2009) | ||
| 2010 | Palagruža Island (Croatia) | 1 | Dragičević et al. (2011) | ||
| 2012 | Arenzano (Italy) | 1 | Psomadakis et al. (2012) | ||
| 2012 | Terracina (Italy) | 1 | Psomadakis et al. (2012) |
| Family, Genus | Species | Year | Mediterranean Area | Number of Specimens | References |
|---|---|---|---|---|---|
| Trachipteridae, Trachipterus | Trachipterus arcticus | before 1986 | Spanish coast | 1 | Robins and Ray (1986) |
| Trachipteridae, Trachipterus | Trachipterus trachypterus | 1881 | Adriatic Sea (Croatia) | N.R. | Kolombatović (1890) |
| 1888 | Gulf of Trieste (Italy) | 2 | Marcuzzi (1972) | ||
| before 1980 | Grignano (Italy) | 46 | Jardas (1980) | ||
| 1980 | Off Sardinian coast (Italy) | 3 | Cau (1980) | ||
| 1992 | Grignano (Italy) | 1 | Bussani (1992) | ||
| 1992 | Ronek Cape (Slovenia) | 1 | Dulčic and Lipej (1997) | ||
| 1996 | Eastern Levant Sea (Israel) | 1 | Golani (1996) | ||
| 1996 | Stončica (Croatia) | 1 | Dulčic (1996) | ||
| 2000 | Anzio (Italy) | 1 | Psomadakis et al. (2006) | ||
| 2006 | Gulf of Trieste (Italy) | 5 | Borme and Voltolina (2006) | ||
| 2010 | Cephalonia Island (Greece) | 1 | Mytilineou et al. (2013) | ||
| 2010–2013 | Ligurian Sea (Italy) | N.R. | Garibaldi (2015) | ||
| 2016 | Çeşme (Turkey) | 1 | Yapici (2019) | ||
| 2017 | Scanzano Ionico (Italy) | 1 | Tirlongo et al. (2019) | ||
| 2018 | Porto Cesareo (Italy) | 1 | Tirlongo et al. (2019) | ||
| 2018 | Marzamemi (Italy) | 1 | Tirlongo et al. (2019) | ||
| 2018 | Izola (Slovenia) | 1 | Lipej et al. (2018) | ||
| 2018 | Ponza (Italy) | 4 (18) * | Macali et al. (2020) | ||
| 2019 | Pianosa (Italy) | 1 | Tiralongo et al. (2020) | ||
| 2020 | Gulf of Antalya (Turkey) | 1 | Gökoğlu and Özen (2021) | ||
| 2021 | Maliakos Gulf (Greece) | 1 | Kaminas et al. (2021) | ||
| 2021 | Pagasitikos Gulf (Greece) | 1 | Kaminas et al. (2021) | ||
| 2021 | Attica Peninsula (Greece) | 2 | Kaminas et al. (2021) | ||
| 2021 | Kerkira Island (Greece) | 2 | Kaminas et al. (2021) | ||
| Trachipteridae, Zu | Zu cristatus | 1918 | La Spezia (Italy) | 1 | Bonelli (1920) |
| 1954 | Gulf of Tunis (Tunis) | 1 | Postel (1955) | ||
| 1955 | Palma de Mallorca (Spain) | 1 | Oliver (1955) | ||
| 1958 | Gulf of Genova (Italy) | 1 | Tortonese (1958) | ||
| 1969 | Blanes (Spain) | 1 | Ibáñez and Gállego (1974) | ||
| 1976 | Gulf of Genova (Italy) | 1 | Gavagnin (1976) | ||
| 1979 | Off Sardinian coast (Italy) | 1 | Cau (1980) | ||
| before 1980 | Central/northern Adriatic Sea | 16 | Jardas (1980) | ||
| 1980 | Arenys de Mar (Spain) | 1 | Roig and Demestre (1982) | ||
| 1981 | Malgrat de Mar (Spain) | 1 | Roig and Demestre (1982) | ||
| 1998 | Gulf of Castellammare (Italy) | 2 | Bianco et al. (2006) | ||
| 1998–2000 | Duće (Croatia) | eggs | Dulčic (2002) | ||
| 2000 | Anzio (Italy) | 1 | Psomadakis et al. (2006) | ||
| 2003 | Gulf of Genova (Italy) | 2 | Psomadakis et al. (2007) | ||
| 2004 | Vis Island (Croatia) | 1 | Dulčic et al. (2014) | ||
| 2009 | Zadar (Croatia) | 1 | Dulčic et al. (2014) | ||
| 2009 | Mahdia (Tunis) | 1 | Bradai and El Ouaer (2012) | ||
| 2010–2013 | Ligurian Sea (Italy) | N.R. | Garibaldi (2015) | ||
| 2013 | Isla de Cabrera (Spain) | 1 | García–Barcelona et al. (2014) | ||
| 2013 | Hvar Island (Croatia) | 1 | Dulčic et al. (2014) | ||
| 2014 | Isla de Formentera (Spain) | 1 | García–Barcelona et al. (2014) | ||
| 2014 | Vibo Valentia (Italy) | 1 | Zenetos et al. (2015) | ||
| 2015 | San Vito lo Capo (Italy) | 1 | Fasone et al. (2017) | ||
| 2017 | Civitavecchia (Italy) | 1 | Tiralongo et al. (2019) | ||
| 2018 | Ponza (Italy) | 1 | Tiralongo et al. (2019) | ||
| 2018 | Avola (Italy) | 1 | Tiralongo et al. (2019) | ||
| 2018 | Porto Cesareo (Italy) | 2 | Tiralongo et al. (2019) | ||
| 2018 | Briatico (Italy) | 1 | Tiralongo et al. (2020) | ||
| 2019 | Pianosa (Italy) | 1 | Tiralongo et al. (2020) | ||
| 2019 | Capraia (Italy) | 1 | Tiralongo et al. (2020) | ||
| 2019 | Fiumicino (Italy) | 1 | Tiralongo et al. (2020) | ||
| 2021 | Dodecanese Islands (Greece) | 1 | Kaminas et al. (2021) | ||
| 2021 | Lesvos Island (Greece) | 1 | Kaminas et al. (2021) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albano, M.; D’Iglio, C.; Spanò, N.; Fernandes, J.M.d.O.; Savoca, S.; Capillo, G. Distribution of the Order Lampriformes in the Mediterranean Sea with Notes on Their Biology, Morphology, and Taxonomy. Biology 2022, 11, 1534. https://doi.org/10.3390/biology11101534
Albano M, D’Iglio C, Spanò N, Fernandes JMdO, Savoca S, Capillo G. Distribution of the Order Lampriformes in the Mediterranean Sea with Notes on Their Biology, Morphology, and Taxonomy. Biology. 2022; 11(10):1534. https://doi.org/10.3390/biology11101534
Chicago/Turabian StyleAlbano, Marco, Claudio D’Iglio, Nunziacarla Spanò, Jorge Manuel de Oliveira Fernandes, Serena Savoca, and Gioele Capillo. 2022. "Distribution of the Order Lampriformes in the Mediterranean Sea with Notes on Their Biology, Morphology, and Taxonomy" Biology 11, no. 10: 1534. https://doi.org/10.3390/biology11101534