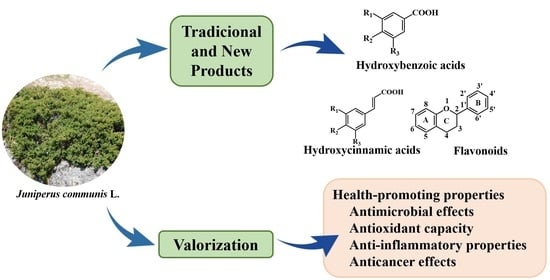
Zimbro (Juniperus communis L.) as a Promising Source of Bioactive Compounds and Biomedical Activities: A Review on Recent Trends
Abstract
:1. Introduction
2. Scientific Classification
3. Phytochemical Composition of Juniperus communis L.
3.1. Carotenoids and Chlorophylls
3.2. Phenolic Compounds
| Gender | Origin | Extract | Total Phenolic Compounds a | Total Flavonoid Content b | Total Anthocyanin Content c | Total Tannin Content b | References | |
|---|---|---|---|---|---|---|---|---|
| Leaves | ||||||||
| J. communis (var. alpina) | n.s. | Serra Da Estrela, Portugal | Methanolic (100%, v/v) | 155.60 | 60.40 | [64] | ||
| J. communis (var. alpina) | n.s. | Yozgat, Turkey | Hydroethanolic (80% ethanol, v/v) | 4.36 | 7.05 | [61] | ||
| J. communis (var. alpina) | n.s. | Yozgat, Turkey | Aqueous | 169.27 | 24.30 | |||
| J. communis (var. communis) | Female | Rhodopes, Bulgaria | Methanolic (80% methanol, v/v) | 132.00 | [60] | |||
| J. communis (var. communis) | Female | Mountain Ozren, near Sarajevo, Bosnia, and Herzegovina | Methanolic (80% methanol, v/v) | 390.89 | 40.22 * | [62] | ||
| J. communis (var. communis) | Male | Mountain Ozren, near Sarajevo, Bosnia, and Herzegovina | Methanolic (80% methanol, v/v) | 544.09 | 48.06 * | |||
| J. communis (var. communis) | n.s. | Nainital, India | Hydroethanolic (70% ethanol, v/v) | 238.78 | [65] | |||
| J. communis (var. communis) | n.s. | Nainital, India | Hexane | 189.65 | ||||
| J. communis (var. communis) | n.s. | Nainital, India | Ethyl acetate | 315.33 | ||||
| J. communis (var. communis) | n.s. | Nainital, India | Aqueous | 205.33 | ||||
| J. communis (var. oblonga pendula) | Male | North Carolina, USA | Methanolic (80% methanol, v/v) | 91.00 | [60] | |||
| J. communis (var. saxatiles) | n.s. | Turkey | Hydroethanolic (80% ethanol, v/v) | 212.10 | [66] | |||
| Berries | ||||||||
| J. communis (var. alpina) | n.s. | Yozgat, Turkey | Hydroethanolic (80% ethanol, v/v) | Ripe berry: 11.92 Unripe berry: 130.92 | Ripe berry: 2.56 Unripe berry: 17.57 | [61] | ||
| J. communis (var. alpina) | n.s. | Yozgat, Turkey | Aqueous | Ripe berry: 4.36 | Ripe berry: 7.05 | |||
| J. communis (var. communis) | North-East Slovakia | Hydroethanolic (70% ethanol, v/v) | Ripe berry: 6.87–42.23 | [67] | ||||
| J. communis (var. communis) | n.s. | Melbourne, Australia | Hydroethanolic (30% ethanol, v/v) | Ripe berry: 9.08 | Ripe berry: 2.25 | Ripe berry: 3.48 * | [68] | |
| J. communis (var. communis) | n.s. | Quebec, Canada | Hydroethanolic (80% ethanol, v/v) | Ripe berry: 99.20 b | Ripe berry: 0.47 | [69] | ||
| J. communis (var. communis) | n.s. | Serra Da Estrela, Portugal | Methanolic (100%, v/v) | Ripe berry: 44.70 | [64] | |||
| J. communis (var. communis) | n.s. | Ağrı, Turkey | Methanolic (100%, v/v) | Ripe berry: 59.17 | [70] | |||
| J. communis (var. n.s.) | n.s. | Pitesti hills, Romania | Hydroethanolic (50% ethanol, v/v) | Ripe berry: 0.19 | Ripe berry: 51.09 d | [40] | ||
| J. communis (var. saxatilis) | n.s. | Yozgat, Turkey | Hydroethanolic (80% ethanol, v/v) | Ripe berry: 21.00 | [66] | |||
| J. communis (var. saxatilis) | n.s. | Ankara, Turkey | Methanolic (100%, v/v) | Ripe berry: 17.64 | [70] | |||
| J. communis (n.s.) | n.s. | Šara mountain in south Serbia | Chloroformic | 189.82 | 27.11 d | [50] | ||
| J. communis (var. n.s.) | n.s. | Šara mountain in south Serbia | Ethanolic | 189.82 | 42.85 d | |||
| J. communis (var. n.s.) | n.s. | Šara mountain in south Serbia | Ethyl acetate | 144.21 | 38.40 d | |||
| Stems | ||||||||
| J. communis (var. alpina) | n.s. | Serra Da Estrela, Portugal | Methanolic (100%, v/v) | 221.30 | 79.30 | [64] | ||
3.2.1. Hydroxycinnamic Acids
3.2.2. Hydroxybenzoic Acids
3.2.3. Flavan-3-ols
3.2.4. Flavonols
3.2.5. Flavones
3.2.6. Coumarins and Flavanones
3.2.7. Anthocyanins
3.3. Volatile Organic Compounds (VOC’s)
4. Biological Potential of Juniperus communis Linnaeus
4.1. Antimicrobial, Antifungal, and Antiparasitic Potential
| Part of the Plant | Origin | Subspecies/Variety | Method | Inhibited Species | References |
|---|---|---|---|---|---|
| Antimicrobial activity | |||||
| Essential oils | |||||
| Berries | Poland | n.s. | Disc diffusion | Staphyllococcus aureus, Serratia marcenscens, Enterobacter cloace, Klebsiella pneumoniae, Pseudomonas aeruginosa, Acinetobacter baumanii, Listeria monocytogenes, and Candida albicans | [89] |
| Needles | Portugal | var. alpina | MIC and MLC | Epidermophyton floccosum, Microsporum canis, M. gypseum, Trichophyton mentagrophytes, T. mentagrophytes var. interdigitale, T. rubrum, and T. verrucosum | [90] |
| Needles and berries | Italy | var. communis | MIC | C. albicans, S. aureus, and P. aeuroginosa | [91] |
| Plant material (leaves and stems) | Iran | n.s. | Disc diffusion | S. aureus, P. aeruginosa, and E. coli | [36] |
| Berries | Slovenia | n.s. | Biofims assay | Campylobacter jejuni, L. monocytogenes | [96] |
| Plant material (undifferentiated) | Slovenia | n.s. | Disc diffusion | S. aureus and C. albicans | [92] |
| Berries | Spain | n.s. | MIC | E. coli, Proteus mirabilis, K. pneumoniae, P. aeruginosa and Morganella morganii, MRSA, and L. monocytogenes | [75] |
| Berries | Portugal | n.s. | MIC and MLC | B. cereus, B. subtilis, E. aerogenes, E. faecalis, E. coli, K. pneumoniae, Proteus mirabilis, P. aeruginosa, Salmonella typhimurium, S. aureus, and C. albicans | [81] |
| Leaves | Croatia | n.s. | Disc diffusion, MIC, and MLC | 16 species of bacteria and 14 species of fungus | [26] |
| Berries | Serbia | n.s. | Disc diffusion, MIC, MLC, and in vivo adhesion assay | S. aureus, MRSA, E. faecalis, L. monocytogenes, E. coli, S. flexneri, S. enteritidis, P. aeruginosa, Aspergillus fumigatus, A. versicolor, A. ochraceus, A. niger, Trichoderma viride, Penicillium funiculosum, P. ochrochloron, and P. verrucosum var. cyclopium | [93] |
| Plant material (leaves and branches) | Egypt | n.s. | MIC | S. aureus, E. coli, and C. albicans | [94] |
| Plant material | Croatia | n.s. | MIC and biofilm assay | Mycobacterium avium, M. intracellulare, and M. gordonae | [97,98] |
| Phenolic-rich extracts | |||||
| Berries | Slovenia | n.s. | Biofilms assay | C. jejuni, L. monocytogenes | [96] |
| Plant material | Italy | n.s. | Disc diffusion and MIC | Actinomyces viscosus, Lactobacillus casei, Streptococcus mutans, S. sobrinus, and general oral microbiota | [99] |
| Berries | Turkey | n.s. | Disc diffusion and MIC | S. epidermidis, S. aureus, B. subtilis, P. aeruginosa, E. coli, and C. albicans | [100] |
| Leaves | Turkey | var. communis and var. saxatilis | MIC | S. aureus | [101] |
| Leaves | Poland | n.s. | Disc diffusion | K. pneumoniae, S. enteritidis, P. aeruginosa, A. baumannii, E. faecium, S. aureus, L. fermentum, Clostridium butyricum, L. monocytogenes, B. coagulans, C. utilis, Aspergillus spp., and Fusarium spp. | [102] |
| Stem (branches) | Italy | var. communis and var. saxatilis | Biofilm formation | S. aureus | [103] |
| Berries | Turkey | var. communis and var. saxatilis | MIC and MLC | S. aureus, S. epidermidis, E. hirae, B. subtilis, E. coli, P. mirabilis, P. aeruginosa, C. albicans, and C. parapsilosis | [70] |
| Leaves | India | n.s. | MIC | E. coli, S. aureus, and K. pneumoniae | [104] |
| Antiparasitic activity | |||||
| Essential oils | |||||
| Stems and leaves | France | n.s. | Radioactive micromethod | Two different strains of Plasmodium falciparum, which were chloroquine-resistant (FcBl) and chloroquine-sensitive (Nigerian) strains | [105] |
4.2. Antioxidant Activity
4.2.1. In Vitro Studies
4.2.2. In Vivo Studies
| Part of the Plant | Origin | Extract | Subspecies/ Variety | Experimental Model | Effect | References |
|---|---|---|---|---|---|---|
| In vitro assay | ||||||
| Berries | Romania | Ethanolic (50% ethanol, v/v) | n.s. | Capacity to scavenge DPPH● | IC50 value of 1.42 µg/mL | [32,40,50,61,67] |
| Serbia | Ethanolic | IC50 value of 28.55 µg/mL | ||||
| Ethyl acetate | IC50 value of 106.44 µg/mL | |||||
| Chloroform | IC50 value of 257.66 µg/mL | |||||
| Poland | Methanolic (70%, methanol v/v) | IC50 values from 6.86 to 13.66 µg/L | ||||
| Essential oils | IC50 varying from 1.27 to 4.25 µg/L | |||||
| Turkey | Methanolic | var. saxatilis | IC50 value of 1.84 mg/mL | |||
| var. communis | IC50 value of 0.63 mg/mL | |||||
| Ethanolic (80% ethanol, v/v) | var. alpina | Inhibitory percentages of 33.25, 34.27, and 36.26% at 0.5, 1, and 2 mg/mL, respectively | ||||
| Aqueous | Inhibitory percentages of 48.40, 63.29, and 82.03% at 0.5, 1, and 2 mg/mL, respectively | |||||
| Poland | Methanolic (70% methanol, v/v) | n.s. | Reducing power potential | Values ranging 6.90 and 10.70 mM FeSO4 × 7H2O | [61,63,67,70] | |
| Essential oils | Values ranging from 0.47 and 1.11 mM FeSO4 × 7H2O | |||||
| Turkey | Methanolic | var. communis | IC50 value of 12.82 mg/mL | |||
| 12.82 ascorbic acid equivalent/mL | ||||||
| var. saxatilis | IC50 value of 64.14 mg/mL | |||||
| 64.14 ascorbic acid equivalent//mL | ||||||
| Ethanolic (80% ethanol, v/v) | var. alpina | Inhibitory percentages of 0.083, 0.095, and 0.203% at 0.5, 1, and 2 mg/mL, respectively | ||||
| Aqueous | Inhibitory percentages of 0.424, 0.689, and 1.371% at 0.5, 1, and 2 mg/mL, respectively | |||||
| Poland | Methanolic (70% methanol, v/v) | n.s. | β-carotene bleaching test | β-carotene inhibitory potential varying from 24.36 to 30.63% | [63] | |
| Essential oils | n.s. | β-carotene inhibitory potential varying from 1.19 to 2.39% | ||||
| Turkey | Methanolic | var. saxatilis | Protect liposomes from lipid peroxidation | IC50 value of 120.07 µg/mL | [70] | |
| var. communis | IC50 value of 4.44 µg/mL | |||||
| Turkey | Methanolic | var. saxatilis | Ferrous ion (Fe2+)-chelating activity | Chelating ability around 30% at 2 mg/mL | [32,61,69] | |
| var. communis | Chelating ability around 15% at 2 mg/mL | |||||
| Ethanolic (80% ethanol, v/v) | var. alpina | Inhibitory percentages of 4.88, 14.86, and 32.82% at 0.5, 1, and 2 mg/mL, respectively | ||||
| Aqueous | Inhibitory percentage of 0.83% at 2 mg/mL | |||||
| Canada | Ethanolic (80% ethanol, v/v) | var. communis | Capacity to scavenge peroxyl radicals | 3876 µM Trolox equivalents at 1 mg/mL | [32,66] | |
| n.s. | Essential oil | n.s. | Capacity to scavenge ABTS•+ species | IC50 value of 10.96 µg/mL | ||
| Turkey | Ethanolic (80% ethanol, v/v) | var. saxatilis | Capacity to scavenge ABTS•+ species | Inhibitory percentages of 42.5%, respectively at 3 mg/mL | ||
| n.s. | Essential oil | n.s. | Capacity to scavenge hydroxyl radicals | IC50 value of 0.0066 µg/mL | [32] | |
| n.s. | Essential oil | n.s. | Capacity to scavenge superoxide anions | IC50 of 0.822 µg/mL | [32,61] | |
| Turkey | Ethanolic (80% ethanol, v/v) | var. alpina | Inhibitory percentages of 20.07, 21.97, and 17.80% at 0.5, 1, and 2 mg/mL, respectively | |||
| Aqueous | var. alpina | Inhibitory percentages of 5.49, 10.61, and 11.17% at 0.5, 1 and 2 mg/mL, respectively | ||||
| Crushed berries | Slovakia | Ethanolic (70% ethanol, v/v) | n.s. | Capacity to scavenge hydroxyl radicals | Inhibitory values varying from 65.59 to 88.12% (recalculated by dry matter (DM), from 3.06 to 5.75%/g DM) | [67] |
| Noncrushed berries | Slovakia | Ethanolic (70% ethanol, v/v) | n.s. | Inhibitory values varying from 15.52 and 32.85% (recalculated by dry matter (DM), from 1.20 to 20.05%/g DM) for | ||
| Unripe berries | Turkey | Ethanolic (80% ethanol, v/v) | var. alpina | Capacity to scavenge superoxide anions | Inhibitory percentages of 14.58, 10.99, and 18.37% at 0.5, 1, and 2 mg/mL, respectively | [61] |
| Capacity to scavenge DPPH● | Inhibitory percentages of 46.21, 57.32, and 73.75% at 0.5, 1, and 2 mg/mL, respectively | [32,61] | ||||
| Capacity to chelate metals | Inhibitory percentages of 6.32, 5.04, and 16.59% at 0.5, 1, and 2 mg/mL, respectively | [61] | ||||
| Ferric-reducing antioxidant power | Inhibitory percentages of 0.288, 0.504, and 0.855% at 0.5, 1, and 2 mg/mL, respectively | |||||
| Leaves | India | Ethanolic (70% ethanol, v/v) | n.s. | Capacity to scavenge DPPH● | IC50 value of 213 µg/mL | [60,61,65,79,107] |
| Aqueous | var. communis | IC50 value of 347 µg/mL | ||||
| Ethyl acetate | IC50 value of 177 µg/mL | |||||
| Turkey | Ethanolic (80% ethanol, v/v) | var. alpina | Inhibitory percentages of 66.62, 83.06, and 91.40% at 0.5, 1, and 2 mg/mL, respectively | |||
| Aqueous | Inhibitory percentages of 34.92, 35.56, and 37.29% at 0.5, 1, and 2 mg/mL, respectively | |||||
| Bulgaria | Methanolic (80% methanol, v/v) | var. Oblonga Pendula | IC50 value of 258 µg/mL | |||
| Serbia | Essential oil | var. communis | IC50 value of 660 µg/mL | |||
| Serbia | Essential oil | var. saxatilis | Potential to chelate metals | IC50 value of 320 µg/mL | [61,107] | |
| India | Ethyl acetate | var. communis | IC50 value of 261 µg/ mL | |||
| Turkey | Acetate | n.s. | Inhibitory effect of 6.05% at 1 mg/mL | |||
| Aqueous | var. alpina | Inhibitory percentages of 9.06, 12.39, and 38.40% at 0.5, 1, and 2 mg/mL, respectively | ||||
| Turkey | Ethanolic (80% ethanol, v/v) | var. alpina | Capacity to scavenge superoxide anions | Inhibitory percentages of 20.26, 25.00, and 25.38% at 0.5, 1, and 2 mg/mL, respectively | [61,71] | |
| Turkey | Ethanolic (80%, ethanol v/v) | var. alpina | Ferric-reducing antioxidant power | Inhibitory percentages of 0.681, 1.278, and 1.971% at 0.5, 1, and 2 mg/mL, respectively | ||
| Aqueous | Inhibitory percentages of 0.121, 0.120, and 0.154% at 0.5, 1, and 2 mg/mL, respectively | |||||
| Serbia | Distilled extracts | var. saxatilis | Reduction capacity of 78.77 mg of ascorbic acid equivalents per g of dry matter | |||
| Serbia | Distilled extracts | var. saxatilis | Lipid-peroxidation inhibitory potential | IC50 value of 540 µg/mL | [71] | |
| Essential oils | IC50 value of 2440 µg/mL | |||||
| Turkey | Ethanolic (80% ethanol, v/v) | var. saxatilis | Capacity to scavenge ABTS•+ species | Inhibitory percentage of 99.5 at 3 mg/mL | [66] | |
| Shoots | Poland | Crude extract | n.s. | Antioxidant-enzyme activity and reactive oxygen species in vitro assays | ↑↑ the activity of intracellular antioxidant enzymes superoxide dismutase and catalase ↓↓ reactive oxygen species | [56] |
| Turkey | Acetone | n.s. | Capacity to chelate metals | Inhibitory percentage of 6.05% at 1 mg/mL | [107] | |
| Ethyl acetate | Inhibitory percentage of 22.59 at 1 mg/mL | |||||
| Ethanolic (75% ethanol, v/v) | Inhibitory percentage of 12.31% at 1 mg/mL | |||||
| Twigs | Spain | Essential oil | n.s. | Peroxy-radical-induced oxidation inhibition | 120 µmol Trolox/gram of essential oil | [53] |
| Hops | Australia | Ethanolic (30% ethanol, v/v) | n.s. | Ferric ion-reducing antioxidant power | 4.17 mg of ascorbic acid equivalents per g | [68] |
| Capacity to scavenge DPPH● | 9.26 mg of ascorbic acid equivalents per g | |||||
| Capacity to scavenge ABTS•+ species | 49.54 mg of ascorbic acid equivalents per g | |||||
| Plant material (twigs, leaves, and berries) | Spain | Essential oil | n.s. | Reducing power assay | IC50 values from 135 to 970 µg/mL | [75] |
| Spain | Essential oil | n.s. | Inhibition of oxidation process | IC50 values from 324.76 to 1563.29 µg/mL | ||
| In vivo assay | ||||||
| Leaves | India | Methanolic | n.s. | Effects on Wistar rats with induced Parkinson’s disease by chlorpromazine for 21 days at a dose of 200 mg/kg | ↑↑ in reduced glutathione ↓↓ levels of TBARS | [19] |
| Romania | Essential oil | n.s. | Effects of juniper volatile oil (1% and 3%) daily inhalation on Amyloid Beta (1–42)-induced oxidative stress in Wistar rats | ↑↑ superoxide dismutase and catalase enzymes, and glutathione peroxidase activity | [85] | |
4.3. Anti-Inflammatory and Antinociceptive Properties
4.3.1. In Vitro Studies
4.3.2. In Vivo Studies
| Part of the Plant | Origin | Extract | Subspecies/ Variety | Experimental Model | Effect | References |
|---|---|---|---|---|---|---|
| Anti-inflammatory and antinociceptive properties | ||||||
| In vitro assay | ||||||
| Plant parts | Sweden | Aqueous | n.s. | Prostaglandin biosynthesis assay Platelet activating factor-induced exocytosis assay | ↓↓ prostaglandins by 55% at 200 µg/mL ↓↓ platelet activating factor-induced exocytosis by 78% at 250 µg/mL | [25] |
| Woods | Austria | Methylene chloride | n.s. | 12(S)-lipoxygenase assay | ↓↓ 12[S]-hydroxy-5,8,10,14-eicosatetraenoic acid by 54.0% at 100 µg/mL, 66.2 and 76.2%, | [88] |
| Berries | Austria | Methylene chloride | n.s. | ↓↓ 12[S]-hydroxy-5,8,10,14-eicosatetraenoic acid by 66.2% at 100 µg/mL | ||
| Ethyl acetate | ↓↓ 12[S]-hydroxy-5,8,10,14-eicosatetraenoic acid by 76.2% at 100 µg/mL | |||||
| Plant material (twigs, leaves, and fruits) | Spain | Essential oil | n.s. | Inhibition of nitric oxide production in lipopolysaccharide-activated murine macrophage RAW 264.7 cells | IC50 values from 23.98 to 84.80 µg/mL | [75] |
| In vivo assays | ||||||
| Berries | Italia | Hydroethanolic (80% ethanol, v/v) | var. communis | Effects on the inhibition of writhing carrageenin foot edema in male Wistar rats after 7 days of treatment at doses of 100 and 200 mg/kg | ↓↓ carrageenin-foot edema by 60% and 79% at 100 and 200 mg/kg, respectively | [113] |
| Turkey | Aqueous | 12.8% inhibition (berries) | [114] | |||
| Berries, leaves, and stems | Turkey | Methanolic | var. communis | 18.5% inhibition (stems) 3.9% inhibition (berries) 18.5% inhibition (leaves) | ||
| Aqueous | var. saxatilis | 9.1% inhibition (berries) 7.8% inhibition (leaves) | ||||
| Methanolic | 30.5% inhibition (berries) 35.2% inhibition (leaves) | |||||
| Aqueous | var. communis | Effects on stimulating response latency in male Swiss albino mice using a hot plate after administration of 100 mg/kg of extract | 4.27% inhibition (stems) 5.36% inhibition (berries) 4.29% inhibition (leaves) | |||
| Methanolic | 4.40% inhibition (stems) 4.11% inhibition (berries) 5.16% inhibition (leaves) | |||||
| Aqueous | var. saxatilis | 3.26% inhibition (stems) 4.32% inhibition (berries) 5.13% inhibition (leaves) | ||||
| Methanolic | 3.13% inhibition (stems) 4.05% inhibition (berries) 5.31% inhibition (leaves) | |||||
| Aqueous | var. communis | Effects on carrageenin-induced hind-paw edema in male Swiss albino mice after 360 min of 100 mg/kg extract administration | 65.9% inhibition (stems) 65.1% inhibition (berries) 65.4% inhibition (leaves) | |||
| Methanolic | 54.3% inhibition (stems) 65.8% inhibition (berries) 54.8% inhibition (leaves) | |||||
| Aqueous | var. saxatilis | 69.6% inhibition (stems) 51.9% inhibition (berries) 53.6% inhibition (leaves) | ||||
| Methanolic | 65.7% inhibition (stems) 43.4% inhibition (berries) 45.3% inhibition (leaves) | |||||
| Methanolic | var. saxatilis | Effects on PGE2-induced hind-paw edema effects in male Swiss albino mice after 360 min of 100 mg/kg extract administration | 17.6% inhibition (stems) 16.5% inhibition (berries) 16.8% inhibition (leaves) | |||
| Leaves | India | Methanolic | n.s. | In vivo study involving different nociceptive assays (acetic acid-induced writhing, formalin and tail-flick tests) in Swiss albino mice at 100 and 200 mg/kg | ↓↓ writhing response and the late phase related with the formalin test Act centrally since the extract and pethidine effects were blocked by naloxone in the tail-flick test | [29] |
| Berries | Romania | Hydroethanolic microemulsions | n.s. | Effects on paw edema in dextran-induced inflammation Wistar rats’ model | ↓↓ paw edema | [40] |
| Berries | Romania | Hydroethanolic microemulsions | n.s. | Kaolin-induced inflammation in Wistar rats’ model | ↓↓ interleukins -1β and 6 expression ↓↓ tumor necrosis factor alfa | |
| Antidiabetic, antihypercholesterolemic and antihyperlipidemic effects | ||||||
| In vitro assays | ||||||
| Fruits | Turkey | Hydroethanolic (80% ethanol, v/v) | var. saxatilis | Capacity to inhibit α-amylase activity | Inhibitory value of 29.8% at 3 mg/mL | [66] |
| Capacity to inhibit α-glucosidase activity | IC50 value of 4.4 µg/mL | |||||
| Leaves | Turkey | Hydroethanolic (80% ethanol, v/v) | var. saxatilis | Capacity to inhibit the α-amylase activity | Inhibitory value of 84.3% at 3/mg/mL | |
| Capacity to inhibit the α-glucosidase activity | IC50 value of 53.6 µg/mL | |||||
| Plant material | United Kingdom | Aqueous | n.s. | Effects on glucose movement | ↓↓ glucose diffusion by 6% at 50 g/L | [124] |
| In vivo assays | ||||||
| Berries | United Kingdom | n.s. | Streptozotocin-induced diabetic mice models for 40 days at doses of 1 g/400 mL | ↓↓ polydipsia Prevent weight losses | [125] | |
| Spain | Aqueous | n.s. | Effects on streptozotocin-induced diabetic rat models after 24 days of treatment at doses of 250 and 500 mg/kg | ↓↓ hypoglycemia in normoglycemic rats | [126] | |
| Effects on streptozotocin-induced diabetic rat models after 24 days of treatment at 125 mg/kg | ↓↓ blood glucose levels and mortality index Prevent weight losses | |||||
| Turkey | Oil dissolved in 0.5% of sodium carboxymethyl cellulose | n.s. | Effects on albino Wistar rats after 30 days of treatment at doses of 50, 100 and 200 mg/kg | ↓↓ total cholesterol, oxidized low-density lipoprotein, alanine aminotransferase, and aspartate transaminase levels ↑↑ blood urea nitrogen and creatinine levels | [33] | |
| Plant | n.s. | Methanolic extracts | n.s. | Effects on streptozotocin-nicotinamide induced diabetic rats after 21 days of treatment at doses of 100 and 200 mg/kg | ↓↓ blood glucose levels, total cholesterol, triglycerides, low-density lipoprotein, and very-low-density lipoprotein cholesterols ↑↑ high-density lipoprotein cholesterol | [31] |
| Herbal preparation also composed of Juniperus. communis | Croatia | Hydroethanolic (60% ethanol, v/v) | n.s. | Effects on alloxan-induced nonobese diabetic NOD mice after 7 days of treatment at 20 mg/kg | ↓↓ glucose and fructosamine levels | [127] |
| Antiproliferative effects | ||||||
| In vitro assays | ||||||
| Berries | Nepal | Aqueous | n.s. | Effects on OECM-1 human gingival squamous cancer cells after 24 h of exposure | Induce apoptosis, exhibiting an IC50 value of 46.20 µg/mL | [128] |
| Plant material | n.s | Aqueous | n.s | Effects on CE81T/VGH human esophageal squamous cell carcinoma after 24, 48 and 72 h of exposure | Induce cell cycle arrest at the G0/G1 phase by regulating the expression of p53/p21 and CDKs/cyclins, triggering cell apoptosis by activating both the extrinsic (Fas/FasL/Caspase 8) and intrinsic (Bcl-2/Bax/Caspase 9) apoptosis pathways IC50 values of 68.41, 64.33, and 60.07 µg/mL after 24, 48, and 72 h of exposure, respectively | [39] |
| Effects on CE48T/VGH human esophageal epidermoid carcinoma after 24, 48, and 72 h of exposure | Induce cell-cycle arrest at the G0/G1 phase, by regulating the expression of p53/p21 and CDKs/cyclins, triggering cell apoptosis by activating both the extrinsic (Fas/FasL/Caspase 8) and intrinsic (Bcl-2/Bax/Caspase 9) apoptosis pathways IC50 values of 69.38, 56.96, and 36.10 µg/mL after 24, 48, and 72 h of exposure, respectively | |||||
| USA | Distilled extracts | Effects on B16/F10 melanoma cells after 24 and 48 h of exposure | Induced apoptosis, decreased angiogenesis and metastasis, and diminished cancer stem-cell expression IC50 values of 27 and 44 µg/mL, after 24 and 48 h of exposure, respectively | [73] | ||
| Leaves | Turkey | Methanolic | n.s. | Effects on C6 rat brain tumor and HeLa human cervix carcinoma cells after 24 h of exposure | IC50 value of 28.43 µg/mL (C6 rat brain tumor) IC50 value of 32.96 µg/mL (HeLa cancer cells) | [72] |
| Aerial parts | Egypt | Methanolic | n.s. | Effects on PC3 human prostate, HCT 116 human colon, and MCF7 breast cancer cells after 24 h of exposure | IC50 value of 23.8 µg/mL (PC3 cancer cells) IC50 value of 37.6 µg/mL (HCT 116 cancer cells) IC50 value of 23.8 µg/mL (MCF7 cancer cells) | [129] |
| Plant material | New Mexico, USA | Aqueous | n.s. | Effects on MCF-7/AZ breast cancer cells after 24 h of exposure | IC50 value of 50 µg/mL | [75,130] |
| Spain | Essential oil | n.s. | Effects on NCI-H460 lung, MCF-7 breast, AGS gastric, and Caco-2 cancer cells after 24 h of exposure | IC50 values varying from 41.99 to 44.87 µg/mL (NCI-H460 cancer cells) IC50 values varying from 30.88 to 163.99 µg/mL (MCF-7 cancer cells) IC50 values varying from 132.68 to 302.86 µg/mL (AGS cancer cells) IC50 values varying from 107.65 to 230.79 µg/mL (Caco-2 cancer cells) | ||
| Berries | Australia | Methanolic | n.s. | Effects on Caco-2 human colorectal and HeLa cervical cancer cells after 12 h of exposure | IC50 value of 1383 µg/mL (Caco-2 cancer cells) IC50 value of 2592 µg/mL (HeLa cancer cells) | [131] |
| Aqueous | n.s. | Effects on Caco-2 human colorectal and HeLa cervical cancer cells after 12 h of exposure | IC50 value of 1516 µg/mL (Caco-2 cancer cells) IC50 value of 2157 µg/mL (HeLa cancer cells) | |||
| Serbia | Essential oil and Distilled extracts | var. saxatilis | Effects on A549 human lung adenocarcinoma epithelial cells after 24 h of treatment after 24 h of exposure | Induced apoptosis and arrested cell cycle in G2/M IC50 value of 69.4 µg/mL (essential oil) IC50 value 1270 µg/mL (distilled extract) | [71] | |
| USA | Distilled extracts | n.s. | Effects on HepG2 human hepatocellular cancer cells after 24, 48, and 72 h of exposure | IC50 values of 48.9, 42.3, and 43.9 µg/mL, after 24, 48, and 72 h of exposure, respectively | [132,133] | |
| Effects on Mahlavu human hepatocellular carcinoma cells after 24, 48, and 72 h of exposure | IC50 values of 64.9, 58.5, and 59.4 µg/mL, after 24, 48, and 72 h of exposure, respectively | |||||
| Effects on J5 human hepatocellular carcinoma cells after 24, 48, and 72 h of exposure | IC50 values of 74.2, 67.2, and 53.2 µg/mL, after 24, 48, and 72 h of exposure, respectively | |||||
| Effects on HT-29 colon cancer cells after 24, 48, and 72 h of exposure | Induced cell-cycle arrest at the G0/G1 phase via regulation of p53/p21 and CDK4/cyclin D1 Induced cell apoptosis via the extrinsic (FasL/Fas/caspase-8) and intrinsic (Bax/Bcl-2/caspase-9) apoptotic pathways IC50 values of 66.71, 60.02, and 54.32 µg/mL, after 24, 48, and 72 h of exposure, respectively | |||||
| Effects on CT-26 colon cancer cells after 24, 48, and 72 h of exposure | Induced cell-cycle arrest at the G0/G1 phase via regulation of p53/p21 and CDK4/cyclin D1 Induced cell apoptosis via the extrinsic (FasL/Fas/caspase-8) and intrinsic (Bax/Bcl-2/caspase-9) apoptotic pathways IC50 values of 27.8, 22.7, and 27.3 µg/mL, after 24, 48, and 72 h of exposure, respectively | |||||
| Leaves and branches | Wyoming, USA | Essential oil | n.s. | Effects on SH-SY5Y human neuroblastoma cells after 24 h of exposure | IC50 value of 53.7 µg/mL | [134] |
| Seed cones | Serbia | Essential oil | var. saxatilis | Effects on HT-29 and HCT116 colon cancer cells after 24 h of exposure | IC50 value 125 µg/mL (HT-29) IC50 value of 62.5 µg/mL (HCT116) | [93] |
| Distilled extracts | IC50 value 625 µg/mL (HT-29) IC50 value of 1250 µg/mL (HCT116) | |||||
| Roots | China | Acetone | n.s. | Effects on N18 neuroblastoma cell lines after 24 and 48 h of exposure | Induced glioma cell-cycle arrest through intrinsic and extrinsic apoptotic pathways IC50 values of 61.11 and 68.94 µg/mL, after 24 and 48 h of exposure, respectively | [135] |
| Effects on DBTRG-05MG, G5T/VGH, GBM8401, GBM8901, and RG2 glioblastoma cell lines after 24 h of exposure | Induced glioma cell-cycle arrest through intrinsic and extrinsic apoptotic pathways IC50 value of 67.04 µg/mL (DBTRG-05MG glioblastoma cells) IC50 value of 63.3 µg/mL (G5T/VGH glioblastoma cells) IC50 value of 57.14 µg/mL (GBM8401glioblastoma cells) IC50 value of 58.45 µg/mL (GBM8901 glioblastoma cells) IC50 value of 69.97 µg/mL (RG2 glioblastoma cells) | |||||
| Effects on DBTRG-05MG, G5T/VGH, GBM8401, GBM8901, and RG2 glioblastoma cell lines after 48 h of exposure | Induced glioma cell-cycle arrest through intrinsic and extrinsic apoptotic pathways IC50 value of 49.46 µg/mL (DBTRG-05MG glioblastoma cells) IC50 value of 67.85 µg/mL (G5T/VGH glioblastoma cells) IC50 value of 46.68 µg/mL (GBM8401glioblastoma cells) IC50 value of 55.49 µg/mL (GBM8901 glioblastoma cells) IC50 value of 53.8 µg/mL (RG2 glioblastoma cells) | |||||
| In vivo assays | ||||||
| Plant | USA | Distilled extracts | n.s. | Effects on melanoma tumor model in C57BL/6 mice after 23 days of treatment at a dose of 200 mg/kg | Cell-cycle arrest at the G0/G1 phase ↓↓ tumor size by 45.2%, B-cell lymphoma-2 (Bcl-2), procaspases 8 and 9 and higher levels of Bcl-2-associated X protein, apoptosis-inducing factor, cell-surface death receptor Fas and Fas ligand when compared to untreated control | [73] |
| Berries | USA | Distilled extracts | n.s. | Effects in BALB/c nude mice injected with HepG2 liver cancer cells at a dose of 200 mg/kg | ↓↓ tumor size ↑↑ lifespan with no or low systemic and pathological toxicity | [132] |
| Effects in female BALB/c mice injected with CT-16 colon cancer cells at a dose of 200 mg/kg | Inhibited proliferation Induced apoptosis No obvious change in body weight or histological morphology of normal organs after treatment | [133] | ||||
| Roots | China | Acetone | n.s. | Effects in male Foxn1 nu/nu mice injected with DBTRG-05MG human glioblastoma cells after 100 days of treatment at a dose of 200 mg/kg | Can penetrate the blood-brain barrier ↓↓ tumor size and the degree of neovascularization ↑↑ PCNA, VEGFR-1, and VEGFR-2 in 44.49%, 5.88%, and 5.85%, respectively, when compared to untreated control | [135] |
| Neuronal effects and anticataleptic activity | ||||||
| In vitro assays | ||||||
| Leaves | Turkey | Hydroethanolic (80% ethanol, v/v) | var. alpina | Capacity to inhibit acetylcholinesterase activity | 10.38% inhibition at 50 µg/mL 24.30% inhibition at 100 µg/mL 32.89% inhibition at 200 µg/mL | [61,107] |
| Ripe Berries | Aqueous | 5.47% inhibition at 100 µg/mL 28.17% inhibition at 200 µg/mL | ||||
| Shoots | Ethyl acetate, ethanolic, and acetone extracts | n.s. | 21.34% inhibition at 100 µg/mL (ethyl acetate extract) 13.46% inhibition at 100 µg/mL (ethanolic extract) 28.43% inhibition at 100 µg/mL (acetone extract) | |||
| Inhibitory percentages varying from 32.34 to 41.97%% inhibition at 100 µg/mL (ethyl acetate extract) Inhibitory percentages varying from 22.29 to 45.45% inhibition at 100 µg/mL (ethanolic extract) Inhibitory percentages varying from 1.91 to 38.55% inhibition at 100 µg/mL (acetone extract) | ||||||
| Leaves | Ethyl acetate, ethanolic, and acetone extracts | n.s. | 20.02% inhibition at 100 µg/mL (ethyl acetate extract) 10.56% inhibition at 100 µg/mL (ethanolic extract) 32.34% inhibition at 100 µg/mL (acetone extract) | |||
| Ripe berries and leaves | Turkey | Aqueous | var. alpina | Capacity to inhibit butyrylcholinesterase activity | 25.87 (berries) and 25.33% (leaves) inhibition at 50 µg/mL 32.57 (berries) and 44.16% (leaves) inhibition at 100 µg/mL 36.97 (berries) and 62.01% (leaves) inhibition at 200 µg/mL | [61,107] |
| Hydroethanolic (80% ethanol, v/v) | 43.68 (berries) and 30.31% (leaves) inhibition at 50 µg/mL 45.19 (berries) and 33.17% (leaves) inhibition at 100 µg/mL 47.55 (berries) and 35.33% (leaves) inhibition at 200 µg/mL | |||||
| Unripe berries | Hydroethanolic (80% ethanol, v/v) | 44.17% inhibition at 50 µg/mL 48.96% inhibition at 100 µg/mL 49.95% inhibition at 200 µg/mL | ||||
| In vivo assays | ||||||
| Leaves | n.s. | Methanolic | n.s. | Effects on Wistar rats with induced Parkinson’s disease by chlorpromazine for 21 days at a dose of 200 mg/kg | ↑↑ locomotor activity ↓↓ motor dysfunctions, including catalepsy and muscle rigidity | [19] |
| Plant material | India | Methanolic | Effects on Wistar rats with induced catalepsy by reserpine 4 h after juniper treatment at a dose of 200 mg/kg | ↓↓ catalepsy activity | [34] | |
| Romania | Essential oil | Effects of juniper volatile oil (1% and 3%) daily inhalation on Amyloid Beta (1–42) male Wistar rat model of Alzheimer’s disease after 21 days of treatment | ↑↑ working memory and reference memory errors within radial arm maze task ↓↓ spontaneous alternations percentage within Y-maze task | [136] | ||
| Effects of juniper volatile oil (1% and 3%) daily inhalation on Amyloid Beta (1–42)-induced oxidative stress in Wistar rats | ↑↑ acetylcholinesterase, superoxide dismutase and catalase activities, and malondialdehyde and protein carbonyl levels ↓↓ glutathione peroxidase-specific activity and the total content of the reduced glutathione | [85] | ||||
| Hepatoprotective effects | ||||||
| In vivo assays | ||||||
| Leaves | India | Ethyl acetate | n.s. | Effects on Wistar albino rats with hepatic damage caused by paracetamol for 14 days at a dose of 200 mg/kg | ↓↓ alkaline phosphatase (−57.41%), direct bilirubin (−30.33%) and total bilirubin (−38.41%), serum alanine aminotransferase (−34.17%), and serum aspartate aminotransferase (−27.58%) when compared to the untreated group Hepatoprotective effects with rearrangement promotion of portal triads and central veins | [65] |
| Stems | n.s. | Petroleum ether, chloroform, and ethanol extracts | n.s. | Effects on rats with hepatic damage caused by carbon tetrachloride | Hepatoprotective activity | [30] |
| Co-combination of berries from juniper and Solanum xanthocarpum | India | Ethanolic | n.s. | Effects on Wistar albino rats with liver toxicity induced by paracetamol and azithromycin for 14 days at a dose of 200 mg/kg | ↓↓ serum glutamate oxaloacetate transaminase (−65.4%), serum glutamate pyruvate transaminase (−59.3%), alkaline phosphatase (66.8%), total bilirubin (62.1%), and liver inflammation Promoting liver tissue’s normal architecture | [3] |
| Tyrosinase inhibitory activity | ||||||
| In vitro assays | ||||||
| Berries | Republic of Korea | Methanolic | n.s. | Capacity to inhibit tyrosinase activity | about 50% inhibition at 100 µg/mL | [137] |
| Renal effects | ||||||
| In vivo assay | ||||||
| Berries | Croatia | Aqueous | n.s. | Daily intake of 10% aqueous infusion, 0.1% of oil (with 0.2% Tween 20 solubilizer) by healthy female Wistar rats | ↑↑ diuresis and urine excretion without loss of electrolytes | [86] |
| Antiurolithiasis effects | ||||||
| In vitro assay | ||||||
| Berries | Iran | Hydroethanolic (50% ethanol, v/v) | n.s. | Capacity to dissolve urinary stone brought out from human kidney at concentrations of 500, 1000, and 2000 µg/mL | Dissolve urinary stones ↓↓ dry powder weight of stones ↑↑ the ratio of calcium oxalate in normal saline aqueous solution plus stone | [138] |
| Gastrointestinal effects | ||||||
| In vivo assays | ||||||
| Leaves | India | Methanolic (80% methanol, v/v) | n.s. | Effects on adult male Wistar albino rats with ulcers induced by aspirin, serotonin, indomethacin, alcohol, and stress at doses of 50 and 100 mg/kg | ↓↓ aspirin, serotonin, indomethacin, alcohol, and stress-induced gastric ulcerations in rats ↑↑ healing rate of acetic acid-induced ulcers in rats | [87] |
| n.s. | Effects on pigs with histamine-induced duodenal lesions at doses of 50 and 100 mg/kg | ↓↓ histamine-induced duodenal lesions in pigs | ||||
| Vessels and trachea protective effects in passive smoking | ||||||
| In vitro assays | ||||||
| Berries | Romania | Aerosols | n.s. | Effects of 3-week juniper aerosols (40 min/day) on female Sprague-Dawley rats firstly exposed to daily passive smoking for 6 weeks | ↓↓ acetylcholine endothelial-dependent relaxation | [139] |
| Oil | n.s. | Effects of 3-week juniper nebulization (20 min/day) on the respiratory tract of rats which firstly exposed to 2 cigarettes per day, 5 days a week for 6 weeks | Bronchodilator effects mediated by nitric oxide | [140] | ||
| Genotoxicity protective effects | ||||||
| In vitro assays | ||||||
| Berries | Romania | Hydroethanolic (50% ethanol, v/v) | n.s. | Capacity to exhibit genoprotective effects against aberrations and abnormalities induced by ethanol on root-tip cells of Allium cepa L. | Can effectively protect chromosomes aberrations | [40] |
4.4. Antidiabetic, Antihypercholesterolemic, and Antihyperlipidemic Effects
4.4.1. In Vitro Studies
4.4.2. In Vivo Studies
4.5. Antiproliferative Effects
4.5.1. In Vitro Studies
4.5.2. In Vivo Studies
4.6. Neuronal Effects and Anticataleptic Activity
4.6.1. In Vitro Studies
4.6.2. In Vivo Studies
4.7. Hepatoprotective Effects
In Vivo Studies
4.8. Tyrosinase Inhibitory Activity
In Vitro Studies
4.9. Renal and Antiurolithiasis Effects
4.9.1. In Vitro Studies
4.9.2. In Vivo Studies
4.10. Gastrointestinal Effects
In Vivo Studies
4.11. Vessels and Trachea Protective Effects in Passive Smoking
In Vivo Studies
4.12. Genotoxicity Protective Effects
In Vitro Studies
4.13. Toxicity Effects
4.13.1. In Vitro Acute Toxicity
4.13.2. Antiprogestogenic and Abortifacient In Vivo Effects
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gezici, S.; Şekeroğlu, N. Current perspectives in the application of medicinal plants against cancer: Novel therapeutic agents. Anticancer Agents Med. Chem. 2019, 19, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Benarba, B.; Pandiella, A. Colorectal cancer and medicinal plants: Principle findings from recent studies. Biomed. Pharmacother. 2018, 107, 408–423. [Google Scholar] [CrossRef] [PubMed]
- Sen, T.; Samanta, S.K. Medicinal plants, human health and biodiversity: A broad review. In Advances in Biochemical Engineering/Biotechnology; Springer: Berlin/Heidelberg, Germany, 2015; Volume 147, pp. 59–110. ISBN 9783662450963. [Google Scholar]
- Dutra, R.C.; Campos, M.M.; Santos, A.R.S.; Calixto, J.B. Medicinal plants in Brazil: Pharmacological studies, drug discovery, challenges and perspectives. Pharmacol. Res. 2016, 112, 4–29. [Google Scholar] [CrossRef] [PubMed]
- Gholipour, S.; Sewell, R.D.E.; Lorigooini, Z.; Rafieian-Kopaei, M. Medicinal plants and atherosclerosis: A review on molecular aspects. Curr. Pharm. Des. 2018, 24, 3123–3131. [Google Scholar] [CrossRef] [PubMed]
- Kirichenko, T.V.; Sukhorukov, V.N.; Markin, A.M.; Nikiforov, N.G.; Liu, P.Y.; Sobenin, I.A.; Tarasov, V.V.; Orekhov, A.N.; Aliev, G. Medicinal plants as a potential and successful treatment option in the context of atherosclerosis. Front. Pharmacol. 2020, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Akkol, K.E.; Dereli, T.G.F.; Sobarzo-Sánchez, E.; Khan, H. Roles of medicinal plants and constituents in gynecological cancer therapy: Current literature and future directions. Curr. Top. Med. Chem. 2020, 20, 1772–1790. [Google Scholar] [CrossRef]
- Singh, G.; Passari, A.K.; Momin, M.D.; Ravi, S.; Singh, B.P.; Kumar, N.S. Ethnobotanical survey of medicinal plants used in the management of cancer and diabetes. J. Tradit. Chin. Med. 2020, 40, 1007–1017. [Google Scholar] [CrossRef]
- Naveed, M.; Majeed, F.; Taleb, A.; Zubair, H.M.; Shumzaid, M.; Farooq, M.A.; Baig, M.M.F.A.; Abbas, M.; Saeed, M.; Changxing, L. A review of medicinal plants in cardiovascular disorders: Benefits and risks. Am. J. Chin. Med. 2020, 48, 259–286. [Google Scholar] [CrossRef] [PubMed]
- Ajebli, M.; Eddouks, M. Phytotherapy of hypertension: An updated overview. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 812–839. [Google Scholar] [CrossRef]
- Shayganni, E.; Bahmani, M.; Asgary, S.; Rafieian-Kopaei, M. Inflammaging and cardiovascular disease: Management by medicinal plants. Phytomedicine 2016, 23, 1119–1126. [Google Scholar] [CrossRef]
- Rouhi-Boroujeni, H.; Heidarian, E.; Rouhi-Boroujeni, H.; Deris, F.; Rafieian-Kopaei, M. Medicinal plants with multiple effects on cardiovascular diseases: A systematic review. Curr. Pharm. Des. 2017, 23, 999–1015. [Google Scholar] [CrossRef] [PubMed]
- Unuofin, J.O.; Lebelo, S.L. Antioxidant effects and mechanisms of medicinal plants and their bioactive compounds for the prevention and treatment of type 2 diabetes: An updated review. Oxid. Med. Cell. Longev. 2020, 1356893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salehi, B.; Ata, A.; Kumar, N.V.A.; Sharopov, F.; Ramírez-Alarcón, K.; Ruiz-Ortega, A.; Ayatollahi, S.A.; Fokou, P.V.T.; Kobarfard, F.; Zakaria, Z.A.; et al. Antidiabetic potential of medicinal plants and their active components. Biomolecules 2019, 9, 551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pathak- Gandhi, N.; Vaidya, A.D.B. Management of Parkinson’s disease in Ayurveda: Medicinal plants and adjuvant measures. J. Ethnopharmacol. 2017, 197, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, P.; Bablon, J.C.; Da Silva, C. A combination of melatonin, vitamin B6 and medicinal plants in the treatment of mild-to-moderate insomnia: A prospective pilot study. Complement. Ther. Med. 2019, 45, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Fejér, J.; Gruľová, D.; Eliašová, A.; Kron, I.; De Feo, V. Influence of environmental factors on content and composition of essential oil from common Juniper ripe berry cones (Juniperus communis L.). Plant Biosyst. 2018, 152, 1227–1235. [Google Scholar] [CrossRef]
- Pers-Kamczyc, E.; Tyrała-Wierucka, Ż.; Rabska, M.; Wrońska-Pilarek, D.; Kamczyc, J. The higher availability of nutrients increases the production but decreases the quality of pollen grains in Juniperus communis L. J. Plant Physiol. 2020, 248, 153156. [Google Scholar] [CrossRef]
- Bais, S.; Gill, N.S.; Kumar, N. Neuroprotective effect of Juniperus communis on chlorpromazine induced Parkinson disease in animal model. Chin. J. Biol. 2015, 2015, 542542. [Google Scholar] [CrossRef] [Green Version]
- Verheyen, K.; Adriaenssens, S.; Gruwez, R.; Michalczyk, I.M.; Ward, L.K.; Rosseel, Y.; Van den Broeck, A.; García, D. Juniperus communis: Victim of the combined action of climate warming and nitrogen deposition? Plant Biol. 2009, 11, 49–59. [Google Scholar] [CrossRef]
- Meireles, C.; Pinto-Gomes, C.; Cano, E. Approach to climatophilous vegetation series of Serra da Estrela (Portugal). Acta Bot. Gall. 2012, 159, 283–287. [Google Scholar] [CrossRef]
- Khare, C.P. Indian Medicinal Plants; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Bais, S.; Gill, N.S.; Rana, N.; Shandil, S. A phytopharmacological review on a medicinal plant: Juniperus communis. Int. Sch. Res. Not. 2014, 2014, 634723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Modnicki, D.; Łabędzka, J. Estimation of the total phenolic compounds in juniper sprouts (Juniperus communis L., Cupressaceae) from different places at the kujawsko-pomorskie province. Herba Pol. 2009, 55, 127–132. [Google Scholar]
- Tunón, H.; Olavsdotter, C.; Bohlin, L. Evaluation of anti-inflammatory activity of some Swedish medicinal plants. Inhibition of prostaglandin biosynthesis and PAF-induced exocytosis. J. Ethnopharmacol. 1995, 48, 61–76. [Google Scholar] [CrossRef]
- Pepeljnjak, S.; Kosalec, I.; Kalodera, Z.; Blažević, N. Antimicrobial activity of juniper berry essential oil (Juniperus communis L., Cupressaceae). Acta Pharm. 2005, 55, 417–422. [Google Scholar] [PubMed]
- Abbassy, M.A.; Marei, G.I.K. Antifungal and chemical composition of essential oils of Juniperus communis L. and Thymus vulgaris L. against two phytopathogenic fungi. J. Appl. Sci. Res. 2013, 9, 4584–4588. [Google Scholar]
- Sati, S.C.; Joshi, S. Antibacterial potential of leaf extracts of Juniperus communis L. from Kumaun Himalaya. Afr. J. Microbiol. Res. 2010, 4, 1291–1294. [Google Scholar]
- Banerjee, S.; Mukherjee, A.; Chatterjee, T.K. Evaluation of analgesic activities of methanolic extract of medicinal plant Juniperus communis Linn. Int. J. Pharm. Pharm. Sci. 2012, 4, 547–550. [Google Scholar]
- Mavin; Garg, G.P. Screening and evaluation of pharmacognostic, phytochemical and hepatoprotective activity of J. communis L. stems. Int. J. Pharma Bio Sci. 2010, 1. [Google Scholar]
- Banerjee, S.; Singh, H.; Chatterjee, T.K. Evaluation of anti-diabetic and anti-hyperlipidemic potential of methanolic extract of Juniperus communis (L.) in streptozotocinnicotinamide induced diabetic rats. Int. J. Pharma Bio Sci. 2013, 4, 10–17. [Google Scholar]
- Höferl, M.; Stoilova, I.; Schmidt, E.; Wanner, J.; Jirovetz, L.; Trifonova, D.; Krastev, L.; Krastanov, A. Chemical composition and antioxidant properties of juniper berry (Juniperus communis L.) essential oil. Action of the essential oil on the antioxidant protection of saccharomyces cerevisiae model organism. Antioxidants 2014, 3, 81–98. [Google Scholar] [CrossRef] [Green Version]
- Akdogan, M.; Koyu, A.; Ciris, M.; Yildiz, K. Anti-hypercholesterolemic activity of Juniperus communis Lynn oil in rats: A biochemical and histopathological investigation. Biomed. Res. 2012, 23, 321–328. [Google Scholar]
- Bais, S.; Gill, N.S.; Rana, N. Effect of Juniperus communis extract on reserpine induced catalepsy. Inventi Impact Ethnopharmacol. 2014, 2014, 1–4. [Google Scholar]
- Raina, R.; Verma, P.K.; Peshin, R.; Kour, H. Potential of Juniperus communis L as a nutraceutical in human and veterinary medicine. Heliyon 2019, 5, e02376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rezvani, S.; Rezai, M.A.; Mahmoodi, N. Analysis and antimicrobial activity of the plant Juniperus communis. Rasayan J. Chem. 2009, 2, 257–260. [Google Scholar]
- Ochocka, J.R.; Asztemborska, M.; Zook, D.R.; Sybilska, D.; Perez, G.; Ossicini, L. Enantiomers of monoterpenic hydrocarbons in essential oils from Juniperus communis. Phytochemistry 1997, 44, 869–873. [Google Scholar] [CrossRef]
- Laouar, A.; Klibet, F.; Bourogaa, E.; Benamara, A.; Boumendjel, A.; Chefrour, A.; Messarah, M. Potential antioxidant properties and hepatoprotective effects of Juniperus phoenicea berries against CCl4 induced hepatic damage in rats. Asian Pac. J. Trop. Med. 2017, 10, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Lee, S.C.; Lai, W.L.; Chang, K.F.; Huang, X.F.; Hung, P.Y.; Lee, C.P.; Hsieh, M.C.; Tsai, N.M. Cell cycle arrest and apoptosis induction by Juniperus communis extract in esophageal squamous cell carcinoma through activation of p53-induced apoptosis pathway. Food Sci. Nutr. 2021, 9, 1088–1098. [Google Scholar] [CrossRef]
- Fierascu, I.; Ungureanu, C.; Avramescu, S.M.; Cimpeanu, C.; Georgescu, M.I.; Fierascu, R.C.; Ortan, A.; Sutan, A.N.; Anuta, V.; Zanfirescu, A.; et al. Genoprotective, antioxidant, antifungal and anti-inflammatory evaluation of hydroalcoholic extract of wild-growing Juniperus communis L. (Cupressaceae) native to Romanian southern sub-Carpathian hills. BMC Complement. Altern. Med. 2018, 18, 3. [Google Scholar] [CrossRef] [Green Version]
- García, D.; Zamora, R.; Gómez, J.M.; Jordano, P.; Hódar, J.A. Geographical variation in seed production, predation and abortion in Juniperus communis throughout its range in Europe. J. Ecol. 2000, 88, 436–446. [Google Scholar] [CrossRef]
- Castro, M.R.; Belo, A.F.; Afonso, A.; Zavattieri, M.A. Micropropagation of Juniperus navicularis, an endemic and rare species from Portugal SW coast. Plant Growth Regul. 2011, 65, 223–230. [Google Scholar] [CrossRef] [Green Version]
- Adams, R.P.; Murata, J.; Takahashi, H.; Schwarzbach, A.E. Taxonomy and evolution of Junperus communis: Insight from DNA sequencing and SNPs. Phytologia 2011, 93, 185–197. [Google Scholar]
- Cano, E.; Musarella, C.M.; Cano-Ortiz, A.; Fuentes, J.C.P.; Torres, A.R.; González, S.D.R.; Gomes, C.J.P.; Quinto-Canas, R.; Spampinato, G. Geobotanical study of the microforests of Juniperus oxycedrus subsp. badia in the central and Southern Iberian Peninsula. Sustainability 2019, 11, 1111. [Google Scholar] [CrossRef] [Green Version]
- Knyazeva, S.G.; Hantemirova, E.V. Comparative analysis of genetic and morpho-anatomical variability of common Juniper (Juniperus communis L.). Russ. J. Genet. 2020, 56, 48–58. [Google Scholar] [CrossRef]
- Ložienė, K.; Rimantas Venskutonis, P. Chapter 56—Juniper (Juniperus communis L.) oils. In Essential Oils in Food Preservation, Flavor and Safety; Academic Press: London, UK, 2016. [Google Scholar] [CrossRef]
- García, D.; Zamora, R.; Hódar, J.A.; Gómez, J.M. Age structure of Juniperus communis L. in the Iberian Peninsula: Conservation of remnant populations in Mediterranean mountains. Biol. Conserv. 1999, 87, 215–220. [Google Scholar] [CrossRef]
- Sequeira, M.; Espírito-santo, M.D. Checklist da Flora de Portugal; ALFA: Lisboa, Portugal, 2011. [Google Scholar]
- Wilson, M. What Juniper Means? Available online: https://www.restaurantnorman.com/what-juniper-means/ (accessed on 22 December 2021).
- Živić, N.; Milošević, S.; Dekić, V.; Dekić, B.; Ristić, N.; Ristić, M.; Sretić, L. Phytochemical and antioxidant screening of some extracts of Juniperus communis L.and Juniperus oxycedrus L. Czech J. Food Sci. 2019, 37, 351–358. [Google Scholar] [CrossRef]
- Bento, C.; Gonçalves, A.C.; Silva, B.; Silva, L.R. Peach (Prunus Persica): Phytochemicals and health benefits. Food Rev. Int. 2020; Ahead-of-print. [Google Scholar] [CrossRef]
- Pott, D.M.; Osorio, S.; Vallarino, J.G. From central to specialized metabolism: An overview of some secondary compounds derived from the primary metabolism for their role in conferring nutritional and organoleptic characteristics to fruit. Front. Plant Sci. 2019, 10, 835. [Google Scholar] [CrossRef] [Green Version]
- Mediavilla, I.; Guillamón, E.; Ruiz, A.; Esteban, L.S. Essential oils from residual foliage of forest tree and shrub species: Yield and antioxidant capacity. Molecules 2021, 26, 3257. [Google Scholar] [CrossRef]
- Bento, C.; Gonçalves, A.C.; Jesus, F.; Simões, M.; Silva, L.R. Phenolic compounds: Sources, properties and applications. In Bioactive Compounds: Sources, Properties and Applications; Porter, R., Parker, N., Eds.; Nova Science Publishers, Inc: New York, NY, USA, 2017; pp. 271–299. ISBN 6312317269. [Google Scholar]
- Fatima, N.; Baqri, S.S.R.; Alsulimani, A.; Fagoonee, S.; Slama, P.; Kesari, K.K.; Roychoudhury, S.; Haque, S. Phytochemicals from Indian ethnomedicines: Promising prospects for the management of oxidative stress and cancer. Antioxidants 2021, 10, 1606. [Google Scholar] [CrossRef]
- Rabska, M.; Robakowski, P.; Ratajczak, E.; Żytkowiak, R.; Iszkuło, G.; Pers-Kamczyc, E. Photochemistry differs between male and female Juniperus communis L. independently of nutritional availability. Trees Struct. Funct. 2021, 35, 27–42. [Google Scholar] [CrossRef]
- Chiorcea-Paquim, A.M.; Enache, T.A.; De Souza Gil, E.; Oliveira-Brett, A.M. Natural phenolic antioxidants electrochemistry: Towards a new food science methodology. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1680–1726. [Google Scholar] [CrossRef] [PubMed]
- Singla, R.K.; Dubey, A.K.; Garg, A.; Sharma, R.K.; Fiorino, M.; Ameen, S.M.; Haddad, M.A.; Al-Hiary, M. Natural polyphenols: Chemical classification, definition of classes, subcategories, and structures. J. AOAC Int. 2019, 102, 1397–1400. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.C.; Rodrigues, M.; Santos, A.O.; Alves, G.; Silva, L.R. Antioxidant status, antidiabetic properties and effects on Caco-2 cells of colored and non-colored enriched extracts of sweet cherry fruits. Nutrients 2018, 10, 1688. [Google Scholar] [CrossRef] [Green Version]
- Olech, M.; Nowak, R.; Ivanova, D.; Tashev, A.; Boyadzhieva, S.; Kalotova, G.; Angelov, G.; Gawlik-Dziki, U. LC-ESI-MS/ MS-MRM profiling of polyphenols and antioxidant activity evaluation of Junipers of different origin. Appl. Sci. 2020, 10, 8921. [Google Scholar] [CrossRef]
- Orhan, N.; Orhan, I.E.; Ergun, F. Insights into cholinesterase inhibitory and antioxidant activities of five Juniperus species. Food Chem. Toxicol. 2011, 49, 2305–2312. [Google Scholar] [CrossRef]
- Mahmutović, I.; Dahija, S.; Bešta-Gajević, R.; Karalija, E. Biological activity of Juniperus communis L. extracts. In Proceedings of the 28th International Scientific-Expert Conference of Agriculture and Food Industry, Sarajevo, Bosnia and Herzegovina, 27–29 September 2017. [Google Scholar]
- Brodowska, A.J.; Smigielski, K.; Nowak, A.; Czyzowska, A.; Otlewska, A. The impact of ozone treatment in dynamic bed parameters on changes in biologically active substances of juniper berries. PLoS ONE 2015, 10, e0144855. [Google Scholar] [CrossRef]
- Luís, Â.; Domingues, F.; Duarte, A.P. Bioactive compounds, RP-HPLC analysis of phenolics, and antioxidant activity of some Portuguese shrub species extracts. Nat. Prod. Commun. 2011, 6, 1863–1872. [Google Scholar] [CrossRef] [Green Version]
- Ved, A.; Gupta, A.; Rawat, A. Antioxidant and hepatoprotective potential of phenol-rich fraction of Juniperus communis Linn. leaves. Pharmacogn. Mag. 2017, 13, 108–113. [Google Scholar] [CrossRef]
- Orhan, N.; Hoşbaş, S.; Orhan, D.D.; Aslan, M.; Ergun, F. Enzyme inhibitory and radical scavenging effects of some antidiabetic plants of Turkey. Iran. J. Basic Med. Sci. 2014, 17, 426–432. [Google Scholar] [CrossRef]
- Fejér, J.; Kron, I.; Grul’ová, D.; Eliašová, A. Seasonal variability of Juniperus communis L. berry ethanol extracts: 1. In vitro hydroxyl radical scavenging activity. Molecules 2020, 25, 4114. [Google Scholar] [CrossRef]
- Tang, J.; Dunshea, F.R.; Suleria, H.A.R. LC-ESI-QTOF/MS characterization of phenolic compounds from medicinal plants (Hops and Juniper Berries) and their antioxidant activity. Foods 2020, 9, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, C.S.; Cuerrier, A.; Lamont, E.; Haddad, P.S.; Arnason, J.T.; Bennett, S.A.L.; Johns, T. Investigating wild berries as a dietary approach to reducing the formation of advanced glycation endproducts: Chemical correlates of in vitro antiglycation activity. Plant Foods Hum. Nutr. 2014, 69, 71–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miceli, N.; Trovato, A.; Dugo, P.; Cacciol, F.; Donato, P.; Marino, A.; Bellinghieri, V.; La Barbera Tommaso, M.; Güvenç, A.; Taviano, M.F. Comparative analysis of flavonoid profile, antioxidant and antimicrobial activity of the berries of Juniperus communis L. var. communis and Juniperus communis L. var. saxatilis Pall. from Turkey. J. Agric. Food Chem. 2009, 57, 6570–6577. [Google Scholar] [CrossRef] [PubMed]
- Vasilijević, B.; Knežević-Vukčević, J.; Mitić-Ćulafić, D.; Orčić, D.; Francišković, M.; Srdic-Rajic, T.; Jovanović, M.; Nikolić, B. Chemical characterization, antioxidant, genotoxic and in vitro cytotoxic activity assessment of Juniperus communis var. saxatilis. Food Chem. Toxicol. 2018, 112, 118–125. [Google Scholar] [CrossRef]
- Sahin Yaglioglu, A.; Eser, F. Screening of some Juniperus extracts for the phenolic compounds and their antiproliferative activities. S. Afr. J. Bot. 2017, 113, 29–33. [Google Scholar] [CrossRef]
- Gao, H.W.; Huang, X.F.; Yang, T.P.; Chang, K.F.; Yeh, L.W.; Hsieh, M.C.; Weng, J.C.; Tsai, N.M. Juniperus communis suppresses melanoma tumorigenesis by inhibiting tumor growth and inducing apoptosis. Am. J. Chin. Med. 2019, 47, 1171–1191. [Google Scholar] [CrossRef]
- Elboughdiri, N.; Ghernaout, D.; Kriaa, K.; Jamoussi, B. Enhancing the extraction of phenolic compounds from juniper berries using the box-behnken design. ACS Omega 2020, 5, 27990–28000. [Google Scholar] [CrossRef]
- Xavier, V.; Finimundy, T.C.; Heleno, S.A.; Amaral, J.S.; Calhelha, R.C.; Vaz, J.; Pires, T.C.S.P.; Mediavilla, I.; Esteban, L.S.; Ferreira, I.C.F.R.; et al. Chemical and bioactive characterization of the essential oils obtained from three Mediterranean plants. Molecules 2021, 26, 7472. [Google Scholar] [CrossRef]
- Kılıç, Ö.; Kocak, A. Volatile constituents of Juniperus communis L., Taxus canadensis Marshall. and Tsuga canadensis (L.) Carr. from Canada. J. Agric. Sci. Technol. B 2014, 4, 135–140. [Google Scholar] [CrossRef]
- Johnson, W. Final report on the safety assessment of Juniperus communis extract, Juniperus oxycedrus extract, Juniperus oxycedrus tar, Juniperus phoenicea extract, and Juniperus virginiana extract. Int. J. Toxicol. 2001, 20, 41–56. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Astatkie, T.; Jeliazkova, E.A.; Heidel, B.; Ciampa, L. Essential oil content, composition and bioactivity of Juniper species in Wyoming, United States. Nat. Prod. Commun. 2017, 12, 201–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajcevic, N.; Dodos, T.; Novakovic, J.; Janackovic, P.; Marin, P. Essential oil composition and antioxidant activity of two Juniperus communis L. varieties growing wild in Serbia. Zb. Matice Srp. Prir. Nauke 2016, 131, 197–205. [Google Scholar] [CrossRef]
- Gonny, M.; Cavaleiro, C.; Salgueiro, L.; Casanova, J. Analysis of Juniperus communis subsp. alpina needle, berry, wood and root oils by combination of GC, GC/MS and 13C-NMR. Flavour Fragr. J. 2006, 21, 99–106. [Google Scholar] [CrossRef] [Green Version]
- Falcão, S.; Bacém, I.; Igrejas, G.; Rodrigues, P.J.; Vilas-Boas, M.; Amaral, J.S. Chemical composition and antimicrobial activity of hydrodistilled oil from Juniper berries. Ind. Crops Prod. 2018, 124, 878–884. [Google Scholar] [CrossRef] [Green Version]
- Chatzopoulou, P.S.; Katsiotis, S.T. Headspace analysis of the volatile constituents from Juniperus communis L. “berries” (cones) grown wild in Greece. Flavour Fragr. J. 2006, 21, 492–496. [Google Scholar] [CrossRef]
- Shahmir, F.; Ahmadi, L.; Mirza, M.; Korori, S.A.A. Secretory elements of needles and berries of Juniperus communis L. ssp. communis and its volatile constituents. Flavour Fragr. J. 2003, 18, 425–428. [Google Scholar] [CrossRef]
- Cavaleiro, C.; Pinto, E.; Gonçalves, M.J.; Salgueiro, L. Antifungal activity of Juniperus essential oils against dermatophyte, Aspergillus and Candida strains. J. Appl. Microbiol. 2006, 100, 1333–1338. [Google Scholar] [CrossRef]
- Cioanca, O.; Hancianu, M.; Mihasan, M.; Hritcu, L. Anti-acetylcholinesterase and antioxidant activities of inhaled Juniper oil on amyloid beta (1–42)-induced oxidative stress in the rat hippocampus. Neurochem. Res. 2015, 40, 952–960. [Google Scholar] [CrossRef]
- Stanić, G.; Samaržija, I.; Blažević, N. Time-dependent diuretic response in rats treated with Juniper berry preparations. Phyther. Res. 1998, 12, 494–497. [Google Scholar] [CrossRef]
- Pramanik, K.C.; Biswas, R.; Bandyopadhyay, D.; Mishra, M.; Ghost, C.; Chatterjee, T.K. Evaluation of anti-ulcer properties of the leaf extract of Juniperus communis L. in animals. J. Nat. Remedies 2007, 7, 207–213. [Google Scholar] [CrossRef]
- Schneider, I.; Gibbons, S.; Bucar, F. Inhibitory activity of Juniperus communis on 12(S)-HETE production in human platelets. Planta Med. 2004, 70, 471–474. [Google Scholar] [CrossRef] [PubMed]
- Filipowicz, N.; Kamiński, M.; Kurlenda, J.; Asztemborska, M.; Ochocka, J.R. Antibacterial and antifungal activity of juniper berry oil and its selected components. Phyther. Res. 2003, 17, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Cabral, C.; Francisco, V.; Cavaleiro, C.; Gonçalves, M.J.; Cruz, M.T.; Sales, F.; Batista, M.T.; Salgueiro, L. Essential oil of Juniperus communis subsp alpina (Suter) Čelak needles: Chemical composition, antifungal activity and cytotoxicity. Phyther. Res. 2012, 26, 1352–1357. [Google Scholar] [CrossRef]
- Angioni, A.; Barra, A.; Russo, M.T.; Coroneo, V.; Dessí, S.; Cabras, P. Chemical composition of the essential oils of Juniperus from ripe and unripe berries and leaves and their antimicrobial activity. J. Agric. Food Chem. 2003, 51, 3073–3078. [Google Scholar] [CrossRef]
- Salamon, I.; Kryvtsova, M.; Bucko, D.; Tarawneh, A.H. Chemical characterization and antimicrobial activity of some essential oils after their industrial large-scale distillation. J. Microbiol. Biotechnol. Food Sci. 2019, 8, 984–988. [Google Scholar] [CrossRef] [Green Version]
- Nikolić, B.; Vasilijević, B.; Ćirić, A.; Mitić-Ćulafić, D.; Cvetković, S.; Džamić, A.; Knežević-Vukčević, J. Bioactivity of Juniperus communis essential oil and post-distillation waste: Assessment of selective toxicity against food contaminants. Arch. Biol. Sci. 2019, 71, 235–244. [Google Scholar] [CrossRef] [Green Version]
- Darwish, R.S.; Hammoda, H.M.; Ghareeb, D.A.; Abdelhamid, A.S.A.; Bellah El Naggar, E.M.; Harraz, F.M.; Shawky, E. Efficacy-directed discrimination of the essential oils of three Juniperus species based on their in-vitro antimicrobial and anti-inflammatory activities. J. Ethnopharmacol. 2020, 259, 112971. [Google Scholar] [CrossRef]
- Ložienė, K.; Švedienė, J.; Paškevičius, A.; Raudonienė, V.; Sytar, O.; Kosyan, A. Influence of plant origin natural α-pinene with different enantiomeric composition on bacteria, yeasts and fungi. Fitoterapia 2018, 127, 20–24. [Google Scholar] [CrossRef]
- Klančnik, A.; Zorko, Š.; Toplak, N.; Kovač, M.; Bucar, F.; Jeršek, B.; Smole Možina, S. Antiadhesion activity of Juniper (Juniperus communis L.) preparations against Campylobacter jejuni evaluated with PCR-based methods. Phyther. Res. 2018, 32, 542–550. [Google Scholar] [CrossRef]
- Peruč, D.; Broznić, D.; Maglica, Ž.; Marijanović, Z.; Karleuša, L.; Gobin, I. Biofilm degradation of nontuberculous mycobacteria formed on stainless steel following treatment with immortelle (Helichrysum italicum) and common juniper (Juniperus communis) essential oils. Processes 2021, 9, 362. [Google Scholar] [CrossRef]
- Peruč, D.; Tićac, B.; Broznić, D.; Maglica, Ž.; Šarolić, M.; Gobin, I. Juniperus communis essential oil limit the biofilm formation of Mycobacterium avium and Mycobacterium intracellulare on polystyrene in a temperature-dependent manner. Int. J. Environ. Health Res. 2020, 32, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Ferrazzano, G.F.; Roberto, L.; Catania, M.R.; Chiaviello, A.; De Natale, A.; Roscetto, E.; Pinto, G.; Pollio, A.; Ingenito, A.; Palumbo, G. Screening and scoring of antimicrobial and biological activities of Italian vulnerary plants against major oral pathogenic bacteria. Evid. Based Complement. Altern. Med. 2013, 316280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kose, M.; Bayraktar, O.; Balta, A. Antioxidant and Antimicrobial Activities of extracts from some selected Mediterranean plant species. Int. J. New Technol. Res. 2016, 2, 263506. [Google Scholar]
- Miceli, N.; Marino, A.; Köroğlu, A.; Cacciola, F.; Dugo, P.; Mondello, L.; Taviano, M.F. Comparative study of the phenolic profile, antioxidant and antimicrobial activities of leaf extracts of five Juniperus L. (Cupressaceae) taxa growing in Turkey. Nat. Prod. Res. 2020, 34, 1636–1641. [Google Scholar] [CrossRef]
- Dziedzinski, M.; Kobus-Cisowska, J.; Szymanowska, D.; Stuper-Szablewska, K.; Baranowska, M. Identification of polyphenols from coniferous shoots as natural antioxidants and antimicrobial compounds. Molecules 2020, 25, 3527. [Google Scholar] [CrossRef]
- Marino, A.; Bellinghieri, V.; Nostro, A.; Miceli, N.; Taviano, M.F.; Güvenç, A.Ş.; Bisignano, G. In vitro effect of branch extracts of Juniperus species from Turkey on Staphylococcus aureus biofilm. FEMS Immunol. Med. Microbiol. 2010, 59, 470–476. [Google Scholar] [CrossRef] [Green Version]
- Rolta, R.; Kumar, V.; Sourirajan, A.; Upadhyay, N.K.; Dev, K. Phytocompounds of three medicinal plants (Juniperus communis, Urtica doica and Coleus forskohilii) of North West Himalayas increases the potency of antibacterial and antiffungal antibiotics. Plant Arch. 2020, 20, 481–489. [Google Scholar]
- Mllhau, G.; Valentin, A.; Benoit, F.; Mallié, M.; Bastide, J.M.; Pélissier, Y.; Bessière, J.M. In vitro antimalarial activity of eight essential oils. J. Essent. Oil Res. 1997, 9, 329–333. [Google Scholar] [CrossRef]
- Van Zyl, R.L.; Seatlholo, S.T.; Van Vuuren, S.F.; Viljoen, A.M. The biological activities of 20 nature identical essential oil constituents. J. Essent. Oil Res. 2006, 18, 129–133. [Google Scholar] [CrossRef]
- Senol, F.S.; Orhan, I.E.; Ustun, O. In vitro cholinesterase inhibitory and antioxidant effect of selected coniferous tree species. Asian Pac. J. Trop. Med. 2015, 8, 269–275. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, A.C.; Bento, C.; Nunes, A.R.; Simões, M.; Alves, G.; Silva, L.R. Multitarget protection of Pterospartum tridentatum phenolic-rich extracts against a wide range of free radical species, antidiabetic activity and effects on human colon carcinoma (Caco-2) cells. J. Food Sci. 2020, 85, 4377–4388. [Google Scholar] [CrossRef] [PubMed]
- Jesus, F.; Gonçalves, A.C.; Alves, G.; Silva, L.R. Exploring the phenolic profile, antioxidant, antidiabetic and anti-hemolytic potential of Prunus avium vegetal parts. Food Res. Int. 2018, 116, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.C.; Lahlou, R.A.; Alves, G.; Garcia-Viguera, C.; Moreno, D.A.; Silva, L.R. Potential activity of Abrantes pollen extract: Biochemical and cellular model studies. Foods 2021, 10, 2084. [Google Scholar] [CrossRef]
- Burits, M.; Asres, K.; Bucar, F. The antioxidant activity of the essential oils of Artemisia afra, Artemisia abyssinica and Juniperus procera. Phyther. Res. 2001, 15, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Emami, S.A.; Abedindo, B.F.; Hassanzadeh-Khayyat, M. Antioxidant activity of the essential oils of different parts of Juniperus excelsa M. Bieb. subsp. excelsa and J. excelsa M. Bieb. subsp. polycarpos (K. Koch) Takhtajan (Cupressaceae). Iran. J. Pharm. Res. 2011, 10, 799–810. [Google Scholar] [PubMed]
- Mascolo, N.; Autore, G.; Capasso, F.; Menghini, A.; Fasulo, M.P. Biological screening of Italian medicinal plants for anti-inflammatory activity. Phyther. Res. 1987, 1, 28–31. [Google Scholar] [CrossRef]
- Akkol, E.K.; Güvenç, A.; Yesilada, E. A comparative study on the antinociceptive and anti-inflammatory activities of five Juniperus taxa. J. Ethnopharmacol. 2009, 125, 330–336. [Google Scholar] [CrossRef]
- Li, X.; Yang, Y.; Li, Y.; Zhang, W.K.; Tang, H. α-Pinene, linalool, and 1-octanol contribute to the topical anti-in fl ammatory and analgesic activities of frankincense by inhibiting COX-2. J. Ethnopharmacol. 2016, 179, 22–26. [Google Scholar] [CrossRef]
- Zhang, T.T.; Hu, T.; Jiang, J.G.; Zhao, J.W.; Zhu, W. Antioxidant and anti-inflammatory effects of polyphenols extracted from Ilex latifolia Thunb. RSC Adv. 2018, 8, 7134–7141. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.Y.; Tang, F.D.; Mao, G.G.; Bian, R.L. Effect of α-pinene on nuclear translocation of NF-κB in THP-1 cells. Acta Pharmacol. Sin. 2004, 25, 480–484. [Google Scholar]
- Limtrakul, P.; Yodkeeree, S.; Pitchakarn, P.; Punfa, W. Suppression of inflammatory responses by black rice extract in RAW 264.7 macrophage cells via downregulation of NF-kB and AP-1 signaling pathways. Asian Pac. J. Cancer Prev. 2015, 16, 4277–4283. [Google Scholar] [CrossRef] [Green Version]
- Zielińska, D.Z.; Zieliński, H.Z.; Giménez-Bastida; Laparra-Llopis, J.M.; Giménez-Bastida, J.A. Caffeic acid modulates processes associated with intestinal inflammation. Nutrients 2021, 13, 554. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.C.; Nunes, A.R.; Falcão, A.; Alves, G.; Silva, L.R. Dietary effects of anthocyanins in human health: A comprehensive review. Pharmaceuticals 2021, 14, 690. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.O.; Min, K.J.; Kwon, T.K.; Um, B.H.; Moreau, R.A.; Choi, S.W. Anti-inflammatory activity of hydroxycinnamic acid derivatives isolated from corn bran in lipopolysaccharide-stimulated Raw 264.7 macrophages. Food Chem. Toxicol. 2012, 50, 1309–1316. [Google Scholar] [CrossRef] [PubMed]
- Byung, H.K.; Sung, M.C.; Reddy, A.M.; Yeong, S.K.; Kyung, R.M.; Kim, Y. Down-regulatory effect of quercitrin gallate on nuclear factor-κB-dependent inducible nitric oxide synthase expression in lipopolysaccharide-stimulated macrophages RAW 264.7. Biochem. Pharmacol. 2005, 69, 1577–1583. [Google Scholar] [CrossRef]
- Bais, S.; Abrol, N.; Prashar, Y.; Kumari, R. Modulatory effect of standardised amentoflavone isolated from Juniperus communis L. agianst Freund’s adjuvant induced arthritis in rats (histopathological and X Ray anaysis). Biomed. Pharmacother. 2017, 86, 381–392. [Google Scholar] [CrossRef]
- Gallagher, A.M.; Flatt, P.R.; Duffy, G.; Abdel-Wahab, Y.H.A. The effects of traditional antidiabetic plants on in vitro glucose diffusion. Nutr. Res. 2003, 23, 413–424. [Google Scholar] [CrossRef]
- Swanston-Flatt, S.K.; Day, C.; Bailey, C.J.; Flat, P.R. Traditional plant treatments for diabetes. Studies in normal and streptozotocin diabetic mice. Diabetologia 1990, 33, 462–464. [Google Scholar] [CrossRef]
- Medina, F.S.; Gamez, M.J.; Jimenez, I.; Jimenez, J.; Osuna, J.I.; Zarzuelo, A. Hypoglycemic activity of Juniper “berries. ” Planta Med. 1994, 60, 197–200. [Google Scholar] [CrossRef]
- Petlevski, R.; Hadžija, M.; Slijepčevič, M.; Juretič, D. Effect of “antidiabetis” herbal preparation on serum glucose and fructosamine in NOD mice. J. Ethnopharmacol. 2001, 75, 181–184. [Google Scholar] [CrossRef]
- Lee, C.C.; Hsiao, C.Y.; Lee, S.C.; Huang, X.F.; Chang, K.F.; Lee, M.S.; Hsieh, M.C.; Tsai, N.M. Suppression of oral cancer by induction of cell cycle arrest and apoptosis using Juniperus communis extract. Biosci. Rep. 2020, 40, BSR20202083. [Google Scholar] [CrossRef] [PubMed]
- Ghaly, N.S.; Mina, S.A.; Younis, N.A.H. In vitro cytotoxic activity and phytochemical analysis of the aerial parts of J. Communis L. cultivated in Egypt. J. Pharm. Sci. Res. 2016, 8, 128–131. [Google Scholar]
- Van Slambrouck, S.; Daniels, A.L.; Hooten, C.J.; Brock, S.L.; Jenkins, A.R.; Ogasawara, M.A.; Baker, J.M.; Adkins, G.; Elias, E.M.; Agustin, V.J.; et al. Effects of crude aqueous medicinal plant extracts on growth and invasion of breast cancer cells. Oncol. Rep. 2007, 17, 1487–1492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez, A.; Cock, I.E. The therapeutic properties of Juniperus communis L.: Antioxidant capacity, bacterial growth inhibition, anticancer activity and toxicity. Pharmacogn. J. 2016, 8, 273–280. [Google Scholar] [CrossRef] [Green Version]
- Huang, N.C.; Huang, R.L.; Huang, X.F.; Chang, K.F.; Lee, C.J.; Hsiao, C.Y.; Lee, S.C.; Tsai, N.M. Evaluation of anticancer effects of Juniperus communis extract on hepatocellular carcinoma cells in vitro and in vivo. Biosci. Rep. 2021, 41, BSR20211143. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.L.; Lee, S.C.; Chang, K.F.; Huang, X.F.; Li, C.Y.; Lee, C.J.; Wu, C.Y.; Hsu, H.J.; Tsai, N.M. Juniperus communis extract induces cell cycle arrest and apoptosis of colorectal adenocarcinoma in vitro and in vivo. Braz. J. Med. Biol. Res. 2021, 54, 1–11. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Caputo, L.; De Martino, L.; Gruľová, D.; Zheljazkov, V.Z.; De Feo, V.; Camele, I. Biological investigations of essential oils extracted from three Juniperus species and evaluation of their antimicrobial, antioxidant and cytotoxic activities. J. Appl. Microbiol. 2020, 129, 1261–1271. [Google Scholar] [CrossRef]
- Tsai, W.C.; Tsai, N.M.; Chang, K.F.; Wang, J.C. Juniperus communis extract exerts antitumor effects in human glioblastomas through blood-brain barrier. Cell. Physiol. Biochem. 2018, 49, 2443–2462. [Google Scholar] [CrossRef]
- Cioanca, O.; Mircea, C.; Trifan, A.; Aprotosoaie, A.C.; Hritcu, L.; Hǎncianu, M. Improvement of amyloid-β-induced memory deficits by Juniperus communis L. volatile oil in a rat model of Alzheimer’s disease. Farmacia 2014, 62, 514–520. [Google Scholar]
- Jegal, J.; Park, S.A.; Chung, K.W.; Chung, H.Y.; Lee, J.; Jeong, E.J.; Kim, K.H.; Yang, M.H. Tyrosinase inhibitory flavonoid from Juniperus communis fruits. Biosci. Biotechnol. Biochem. 2016, 80, 2311–2317. [Google Scholar] [CrossRef] [Green Version]
- Barzegarnejad, A.; Azadbakht, M.; Emadian, O.; Ahmadi, M. Effect of some fractions of the extract of Juniperus communis fruit on solving kidney stones in vitro. J. Maz. Univ. Med. Sci. 2013, 23, 145–152. [Google Scholar]
- Sturza, A.; Pleşa, C.; Ordodi, V.; Noveanu, L.; Mirica, N.; Fira-Mladinescu, O.; Lupea, A.X.; Muntean, D. Vascular responses of Juniperus communis aerosols in aortic rings isolated from rats subjected to passive smoking. Ann. Rom. Soc. Cell Biol. 2011, 16, 178–181. [Google Scholar]
- Pleşa, C.; Ordodi, V.L.; Noveanu, L.; Vasile, L.; Ardelean, A.; Lupea, A.X. Effects of Juniperus communis aerosols on trachea and lung from rat subjected to passive smoking. Stud. Univ. Vasile Goldis Ser. Stiint. Vietii Life Sci. Ser. 2011, 21, 319–328. [Google Scholar]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Lantto, T.A.; Laakso, I.; Dorman, H.J.D.; Mauriala, T.; Hiltunen, R.; Kõks, S.; Raasmaja, A. Cellular stress and p53-associated apoptosis by Juniperus communis L. berry extract treatment in the human SH-SY5Y neuroblastoma cells. Int. J. Mol. Sci. 2016, 17, 1113. [Google Scholar] [CrossRef] [Green Version]
- De Marino, S.; Cattaneo, F.; Festa, C.; Zollo, F.; Iaccio, A.; Ammendola, R.; Incollingo, F.; Iorizzi, M. Imbricatolic acid from Juniperus communis L. prevents cell cycle progression in CaLu-6 cells. Planta Med. 2011, 77, 1822–1828. [Google Scholar] [CrossRef] [Green Version]
- Benzina, S.; Harquail, J.; Jean, S.; Beauregard, A.-P.; Colquhoun, C.; Carroll, M.; Bos, A.; Gray, C.; Robichaud, G. Deoxypodophyllotoxin isolated from Juniperus communis induces apoptosis in breast cancer cells. Anticancer Agents Med. Chem. 2014, 15, 79–88. [Google Scholar] [CrossRef]
- Singh, H.; Prakash, A.; Kalia, A.N.; Majeed, A.B.A. Synergistic hepatoprotective potential of ethanolic extract of Solanum xanthocarpum and Juniperus communis against paracetamol and azithromycin induced liver injury in rats. J. Tradit. Complement. Med. 2016, 6, 370–376. [Google Scholar] [CrossRef] [Green Version]
- Tisserand, R.; Young, R. Essential oil Safety: A Guide for Health Care Professionals, 2nd ed.; Tisserand, R., Young, R., Eds.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2013; ISBN 9780443062414. [Google Scholar]
- Pathak, S.; Tewari, R.K.; Prakash, A.O. Hormonal properties of ethanolic extract of Juniperus communis Linn. Anc. Sci. Life 1990, 10, 106–113. [Google Scholar]
- Agrawal, O.P.; Bharadwaj, S.; Mathur, R. Antifertility effects of fruits of Juniperus communis. J. Med. Plant Res. 1980, 98–101. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonçalves, A.C.; Flores-Félix, J.D.; Coutinho, P.; Alves, G.; Silva, L.R. Zimbro (Juniperus communis L.) as a Promising Source of Bioactive Compounds and Biomedical Activities: A Review on Recent Trends. Int. J. Mol. Sci. 2022, 23, 3197. https://doi.org/10.3390/ijms23063197
Gonçalves AC, Flores-Félix JD, Coutinho P, Alves G, Silva LR. Zimbro (Juniperus communis L.) as a Promising Source of Bioactive Compounds and Biomedical Activities: A Review on Recent Trends. International Journal of Molecular Sciences. 2022; 23(6):3197. https://doi.org/10.3390/ijms23063197
Chicago/Turabian StyleGonçalves, Ana C., José David Flores-Félix, Paula Coutinho, Gilberto Alves, and Luís R. Silva. 2022. "Zimbro (Juniperus communis L.) as a Promising Source of Bioactive Compounds and Biomedical Activities: A Review on Recent Trends" International Journal of Molecular Sciences 23, no. 6: 3197. https://doi.org/10.3390/ijms23063197