Molecular characterization and phylogenetic analyses of Lophodermella needle pathogens (Rhytismataceae) on Pinus species in the USA and Europe
- Published
- Accepted
- Received
- Academic Editor
- Simon Shamoun
- Subject Areas
- Ecology, Evolutionary Studies, Mycology, Plant Science, Forestry
- Keywords
- Pine, Needle, Phylogeny, Pathogen, Morphology, Molecular
- Copyright
- © 2021 Ata et al.
- Licence
- This is an open access article distributed under the terms of the Open Government License.
- Cite this article
- 2021. Molecular characterization and phylogenetic analyses of Lophodermella needle pathogens (Rhytismataceae) on Pinus species in the USA and Europe. PeerJ 9:e11435 https://doi.org/10.7717/peerj.11435
Abstract
Increasing prevalence of conifer needle pathogens globally have prompted further studies on pathogen identification and a better understanding of phylogenetic relationships among needle pathogens. Several Lophodermella species can be aggressive pathogens causing needle cast in natural pine forests in the USA and Europe. However, their relationships with other Rhytismataceae species have historically been based on similarities of only limited phenotypic characters. Currently, no molecular studies have been completed to elucidate their relationships with other Lophodermella needle pathogens. This study collected and sequenced three gene loci, namely: internal transcribed spacer, large ribosomal subunit, and translation elongation factor 1-alpha, from five Lophodermella needle pathogens from North America (L. arcuata, L. concolor, L. montivaga) and Europe (L. conjuncta and L. sulcigena) to distinguish phylogeny within Rhytismatacaeae, including Lophophacidium dooksii. Phylogenetic analyses of the three loci revealed that all but L. conjuncta that were sampled in this study consistently clustered in a well-supported clade within Rhytismataceae. The multi-gene phylogeny also confirmed consistent nesting of L. dooksii, a needle pathogen of Pinus strobus, within the clade. Potential synapomorphic characters such as ascomata position and ascospore shape for the distinct clade were also explored. Further, a rhytismataceous species on P. flexilis that was morphologically identified as L. arcuata was found to be unique based on the sequences at the three loci. This study suggests a potential wider range of host species within the genus and the need for genetic characterization of other Lophodermella and Lophophacidium species to provide a higher phylogenetic resolution.
Introduction
Conifer needle diseases are becoming increasingly prevalent due to several factors such as climate change and introduction to new hosts (Woods, Coates & Hamann, 2005; Lee et al., 2017; Wyka et al., 2017; Brodde et al., 2018). Native needle pathogens emerge as they move into novel geographic areas while others are increasing in incidence due to faster sporulation enhanced by warmer and wetter conditions (Barnes et al., 2014; Gray et al., 2013; Rodas et al., 2016; Welsh, Lewis & Woods, 2014). Recent examples of needle diseases with enhanced severity include Dothistroma needle blight (Woods, 2014), Swiss needle cast and Cedar leaf blight (Gray et al., 2013), and white pine needle damage (Wyka et al., 2018; Broders et al., 2015).
In the western region of USA, an increasing prevalence of native Lophodermella needle pathogens, which may be attributed to climate change, were observed (Worrall, Marchetti & Mask, 2012) in Pinus contorta and P. flexilis. These two pine hosts are naturally dominant and ecologically important species along the Rocky Mountain Region (Lotan & Critchfield, 1990; Schoettle, 2004). Two needle cast epidemics caused by L. concolor and L. montivaga were recorded on P. contorta (Worrall, Marchetti & Mask, 2012) while increased frequency of L. arcuata infection was observed in patches of limber pine (P. flexilis) stands. Meanwhile, in Europe, heavy infection of L. sulcigena and L. conjuncta on European mountain pine (P. mugo) along the Swiss Alps were recorded in 2018 (Beenken, 2019). Despite increasing incidence, there are no wide scale assessments on the impact of Lophodermella pathogens in natural pine stands amidst climate change. Past surveys reported short outbreaks or minor incidence of Lophodermella species such as L. cerina in southern USA, L. morbida in the western USA, L. maureri in Mexico, and L. orientalis in Asia (Czabator et al., 1971; Darker, 1932; Minter, 1988b; Minter, 1993) but there are no recent surveys nor reports about their increasing incidence in these regions.
Thus far, only nine species belong to Lophodermella genus, including L. arcuata, L. cerina, L. concolor, L. maureri, L. montivaga and L. morbida in North America, L. conjuncta and L. sulcigena in Europe, and L. orientalis in Asia (MycoBank Database, 2016). Lophodermella species (Rhytismataceae) are distinguished by their subhypodermal ascomata, clavate ascospores surrounded by mucilaginous sheath, and wider asci than the closely related genus Lophodermium (Darker, 1967). While morphometric descriptions are clear in the literature, identification and differentiation among these Lophodermella species is challenging. This may be attributed to similarities in early symptoms of the disease, highly variable morphometric features at different developmental stages and mounting medium, secondary fungal invasion, and lack of ideally mature specimens (Worrall, Marchetti & Mask, 2012). Based on morphological characteristics there have been doubts on disease reports of L. sulcigena on P. radiata, P. halepensis and P. contorta while other diseases still need verification, such as the occurrence of L. montivaga on P. monticola and P. flexilis (Millar, 1984).
Molecular characterization could help resolve classification of species closely related to Lophodermella such as the case of Lophophacidium dooksii on needles of five-needle Pinus strobus. In 1984, the newly described L. dooksii was classified under Phacidiaceae due to the lack of morphological characteristics distinctive of Rhytismataceae (Corlett & Shoemaker, 1984). However, recent internal transcribed spacer (ITS) phylogenetic studies and morphology suggest Lophophacidium dooksii is closely related to L. arcuata (Laflamme et al., 2015; Ekanayaka et al., 2019). Following the phylogenetic evidence, Ekanayaka (2019) reclassified L. dooksii to Rhytismataceae, but the phylogenetic relationship of L. dooksii and L. arcuata with other Lophodermella species is still unclear.
The lack of molecular information on Lophodermella spp. makes it difficult to resolve intra- and interspecific phylogenetic relationships. Currently, out of the nine known Lophodermella species, only the ITS sequence of L. arcuata represents the genus in fungal genetic databases (i.e., NCBI-nr, UNITE, DNA Data Bank of Japan). As emerging pathogens, molecular studies on Lophodermella are important for pathogen identification. These will elucidate phylogenetic relationship of Lophodermella with other rhytismataceaous species. These will also aid in assessing the diversity and impact of emerging or invasive disease threats in conifer forest and will provide insights on fungal biology and evolution of traits. This study aims to fill this gap by analyzing the three-loci phylogeny of Lophodermella species that cause emerging needle cast diseases in western USA and Europe which include L. arcuata, L. concolor, L. conjuncta, L. montivaga, and L. sulcigena. We test monophyly of this genus by including other genera within Rhytismataceae and by using molecular phylogenies to guide the identification of shared and unique traits among Lophodermella species for genus and species delineation.
Materials & Methods
Sampling and morphology
Sampling was conducted in known geographic distributions of L. arcuata, L. concolor, L. montivaga and L. dooksii in the USA. Similarly, L. sulcigena and L. concolor samples were collected from their known distributions in Europe. Needles from 32 P. contorta trees from natural stands infected with L. montivaga and/or L. concolor were collected in June and August 2018 across 12 sites within Gunnison National Forest, Colorado, USA (Table 1). Lophodermella arcuata on P. flexilis stands were collected from Rocky Mountain National Park, Colorado, USA in June 2018 and July 2019 while the eastern white pine (P. strobus) needles symptomatic of L. dooksii were collected from natural stands in Maine, USA in May 2019. Collections were approved by the USDA Forest Service, Forest Health Protection. Needles of the P. mugo infected with L. sulcigena and L. conjuncta were collected in the Swiss and Austrian Alps in 2018 (Table 1). Needles were placed into separate paper bags and stored at 4 °C until DNA extraction.
| Sample ID | Location | Host | Collection Date | Collectors | GenBank Accession Number; (Genotype) | ||
|---|---|---|---|---|---|---|---|
| ITS | LSU | TEF1-α | |||||
| Lophodermella concolor(Dearn.) Darker | |||||||
| CS6C | CS, GNF, CO, USA | Pinus contorta | 12 June 2018 | JE Stewart, JP Ata, KS Burns, SB Marchetti, JJ Worrall | MN937619; (1) | MN937581; (1) | MN937651; (1) |
| CS9C | CS, GNF, CO, USA | P. contorta | 12 June 2018 | ” | MN937612; (1) | MN937579; (1) | MN937650; (1) |
| FS6C | FS, GNF, CO, USA | P. contorta | 12 June 2018 | ” | MN937618; (1) | MN937582; (1) | MN937647; (1) |
| FS8C | FS, GNF, CO, USA | P. contorta | 12 June 2018 | ” | MN937610; (2) | MN937580; (1) | MN937653; (1) |
| LP7C | LP, GNF, CO, USA | P. contorta | 12 June 2018 | ” | MN937621; (1) | MN937588; (3) | MN937654; (1) |
| LV7C | LV, GNF, CO, USA | P. contorta | 14 June 2018 | ” | MN937620; (1) | MN937575; (1) | MN937657; (1) |
| LV8C | LV, GNF, CO, USA | P. contorta | 12 June 2018 | ” | MN937615; (1) | MN937576; (2) | MN937655; (1) |
| PT2C | PT, GNF, CO, USA | P. contorta | 14 June 2018 | ” | MN937616; (1) | MN937577; (1) | MN937646; (1) |
| PT3C | PT, GNF, CO, USA | P. contorta | 14 June 2018 | ” | MN937614; (1) | MN937583; (1) | MN937652; (1) |
| SR3C | SR, GNF, CO, USA | P. contorta | 13 June 2018 | ” | MN937617; (1) | MN937578; (1) | MN937649; (1) |
| SR6C | SR, GNF, CO, USA | P. contorta | 13 June 2018 | ” | MN937613; (1) | MN937584; (1) | MN937648; (1) |
| OJ11C | OJ, GNF, CO, USA | P. contorta | 13 June 2018 | ” | MN937611; (1) | MN937574; (1) | MN937656; (1) |
| Lophodermella montivagaPetrak | |||||||
| CU1M | CU, GNF, CO, USA | P. contorta | 14 June 2018 | ” | MN937633; (1) | MN937586; (1) | MN937669; (1) |
| LVP2M | LV, GNF, CO, USA | P. contorta | 14 June 2018 | ” | MN937634; (1) | MT906358; (1) | – |
| LVP3M | LV, GNF, CO, USA | P. contorta | 14 June 2018 | ” | MN937635; (1) | MN937598; (1) | MN937672; (1) |
| NC2M | NC, GNF, CO, USA | P. contorta | 14 June 2018 | ” | MN937625; (1) | MN937592; (1) | MN937667; (1) |
| NC6M | NC, GNF, CO, USA | P. contorta | 14 June 2018 | ” | MN937626; (1) | MN937601; (1) | MN937674; (1) |
| NC8M | NC, GNF, CO, USA | P. contorta | 14 June 2018 | ” | MN937627; (1) | MN937593; (1) | MN937671; (1) |
| NC9M | NC, GNF, CO, USA | Pinus contorta | 14 June 2018 | ” | MN937636; (1) | – | MN937668; (1) |
| NC10M | NC, GNF, CO, USA | P. contorta | 14 June 2018 | ” | MN937637; (1) | – | MT919224; (1) |
| OJ3M | OJ, GNF, CO, USA | P. contorta | 13 June 2018 | ” | MN937641; (1) | – | MT919226; (1) |
| PT6M | PT, GNF, CO, USA | P. contorta | 14 June 2018 | ” | MN937640; (2) | MN937594; (1) | MN937661; (1) |
| PT8M | PT, GNF, CO, USA | P. contorta | 14 June 2018 | ” | MN937628; (1) | MN937602; (1) | MN937660; (1) |
| PT9M | PT, GNF, CO, USA | P. contorta | 14 June 2018 | ” | MN937642; (1) | MN937587; (1) | – |
| PT10M | PT, GNF, CO, USA | P. contorta | 14 June 2018 | ” | MN937622; (1) | MN937591; (1) | MN937670; (1) |
| PT11M | PT, GNF, CO, USA | P. contorta | 14 June 2018 | ” | MN937630; (1) | MN937595; (1) | MN937663; (1) |
| SR9M | SR, GNF, CO, USA | P. contorta | 13 June 2018 | ” | MN937643; (3) | – | MN937659; (1) |
| TC1M | TC, GNF, CO, USA | P. contorta | 14 June 2018 | ” | MN937631; (1) | MN937596; (1) | – |
| TC3M | TC, GNF, CO, USA | P. contorta | 14 June 2018 | ” | MN937632; (1) | MN937597; (1) | MN937666; (1) |
| TC9M | TC, GNF, CO, USA | P. contorta | 14 June 2018 | ” | MN937629; (1) | MN937599; (1) | MN937673; (1) |
| TL8M | TL, GNF, CO, USA | P. contorta | 21 August 2018 | SB Marchetti | MN937638; (1) | MN937600; (1) | MN937662; (1) |
| TL9M | TL, GNF, CO, USA | P. contorta | 21 August 2018 | SB Marchetti | MN937639; (2) | – | MT919225; (1) |
| Lophodermellasp. | |||||||
| RMNP_01 | RMNP, CO, USA | Pinus flexilis | 05 July 2018 | KS Burns | MN937645 | MN937590 | MN937665 |
| Lophodermella arcuata(Darker) Darker | |||||||
| RMNP_LU1 | RMNP, CO, USA | P. flexilis | 24 July 2019 | KS Burns | MN937644; (1) | MN937585; (1) | MN937658; (1) |
| RMNP_LU16 | RMNP, CO, USA | P. flexilis | 24 July 2019 | KS Burns | MT906333; (1) | MT906359; (1) | MT919227; (2) |
| Lophophacidium dooksiiCorlett and Shoemaker | |||||||
| MB5 | Massabesic Experimental Forest, ME, USA | Pinus strobus | 03 May 2019 | IA Munck, JE Stewart, JP Ata, A Bergdahl, W Searles | MN937623 | MN937589 | MN937664 |
| Lophodermella sulcigena(Rostr.) Höhn. | |||||||
| PH18_0656 | Canton Ticino, Passo del Lucomagno, CH | Pinus mugo | 10 July 2018 | G Moretti | MN937624 | MN937604 | MN937675 |
| Lophodermella conjuncta(Darker) Darker | |||||||
| PH18_0655 | Canton Grisons, Lenzerheide, CH | P. mugo | 18 April 2018 | M Vanoni | MN937607; (1) | MN937605; (1) | MN937677; (1) |
| PHP19_0986 | Canton Bern, Kandersteg, Oeschi-Forest, CH | P. mugo | 18 June 2018 | J Meyer, L Beenken | MN937609; (2) | MN937606; (1) | MN937676; (1) |
| PHP19_0987 | Tyrol, Scharnitz, Karwendel Valley, AT | P. mugo | 11 June 2018 | T Cech, L. Beenken | MN937608; (3) | MN937603; (1) | MN937678; (1) |
Notes:
- CS
-
Cold Springs Campground
- CU
-
Cumberland
- FS
-
Fisherman Trail
- LP
-
Lodgepole Campground
- LV
-
Lakeview Campground
- NC
-
North Cumberland
- OJ
-
Oh Be Joyful
- PT
-
Pitkin
- SR
-
Slate River
- TC
-
Tincup
- TL
-
Taylor Park
- GNF
-
Gunnison National Forest
- RMNP
-
Rocky Mountain National Park
- CO
-
Colorado
- ME
-
Maine
- USA
-
United States of America
- CH
-
Switzerland
- AT
-
Austria
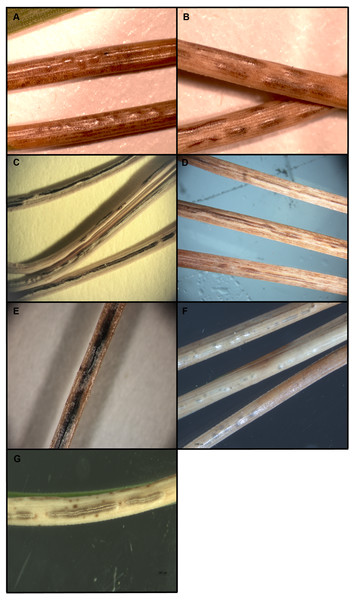
Morphology of the fungal pathogens from randomly selected fresh symptomatic needles was characterized for fungal identification (Fig. 1). Midsections of ascomata were cut using a razor blade and mounted in 3% potassium hydroxide (KOH). Measurements of fruiting structures were taken from mounted materials. Morphological traits common among species based on published descriptions were compared (Table 2; Corlett & Shoemaker, 1984; Darker, 1932; Millar & Minter, 1980; Millar & Minter, 1978; Minter & Millar, 1993a; Worrall, Marchetti & Mask, 2012).
Figure 1: Ascomata of Lophodermella concolor (A) and L. montivaga (B) on Pinus contorta from Gunnison National Forest, Colorado, USA; Lophodermella sp. (C) and Lophodermella arcuata (D) on P. flexilis from Rocky Mountain National Park, Colorado, USA; Lophophacidium dooksii on P. strobus from Massabesic, Maine, USA (E); and L. conjuncta (F) and L. sulcigena (G) on P. mugo from Austria and Switzerland.
| Features | Lophodermella concolor(Dearn.) Darker | Lophodermella montivagaPetrak | Lophodermella arcuata(Darker) Darker | Lophodermella sulcigena(Rostr.) Höhn | Lophodermella conjuncta(Darker) Darker |
|---|---|---|---|---|---|
| (A). Ascomata (hysterothecia) | |||||
| Size (mm) | 0.4–0. 8 × 0.28–0.44 | 0.75–8 × 0.28–0.4 | 0.38–3.13 × 0.25–0.45 | 2–20 × 0.30–0.45 | 0.50–4.0 × 0.20–0.30 |
| Depth (µm) | 200–280 | 220–250 | 210–260 | 200–250 | 140–180 |
| Opening | longitudinal split along stomata | longitudinal split | Longitudinal split along stomata | longitudinal split | longitudinal split |
| Paraphyses | |||||
| Size (µm) | About as long as the asci | Up to 150 ×ca 1 | 120–135 × 0.5–1 | 100–120 × 1 | 135–150 × 1.0–2.0 |
| Gelatinous sheath/ Mucous coat | Present | Present | Present | Present | Absent |
| Septation | Present | Present | Inconspicuous | Present | Present |
| Asci | |||||
| Size (µm) | 120–225 × 15–17 | 120–160 × 12–15 | 110–160 × 14–20 | 110–140 × 13–15 | (100)110–160 × 15–16 |
| Opening mechanism | No obvious pre-formed apical apparatus (small apical hole or split after spores are released) | No obvious pre-formed apical apparatus (small apical hole or split after spores are released) | No obvious pre-formed opening mechanism (small apical hole or split after spores are released) | No obvious pre-formed apical apparatus | No obvious pre-formed apical apparatus |
| Number of spores | 8 | 8 | 8 | 4–8 | 8 |
| Ascospore | |||||
| Size (µm) | 45–60 × (4) 6–8 | 40–50 × 3–4 | 40–50-(95) ×4–6 | 27–40 (65) ×4-5 (6) | (65) 75–90 (100) ×2.5–3.5 |
| Mucilaginous/ gelatinous sheath | Present | Present | Present | Present | Present |
| Hosts (number of needles) | Pinus banksiana (2), P. contorta (2), P. contorta var. murrayana (2), P. sylvestris (2) | Pinus attenuata (3), P. contorta (2), P. sylvestris (2), P. ponderosa (3), P. radiata (3), P. flexilis (5), P. monticola (5) | Pinus albicaulis (5), P. flexilis (5), P. lambertiana (5), P. monticola (5) | Pinus sylvestris (2), P. mugo (2), P. nigra var. maritima (2) | Pinus mugo (2), P. nigra var. Maritima (2), P. sylvestris (2). |
| Distribution | Western USA, Canada | Western USA | Western USA | Europe | Europe |
| Conidiomata | Not observed | Not observed | Not observed | Unknown | Unknown |
| References | (Darker, 1932; Millar, 1984; Minter & Millar, 1993b; Funk, 1985; Worrall, Marchetti & Mask, 2012) | (Darker, 1932; Millar, 1984; Minter & Millar, 1993c; Worrall, Marchetti & Mask, 2012) | (Darker, 1932; Minter & Millar, 1993a) | (Darker, 1932; Millar, 1984; Millar & Minter, 1978, Beenken, 2019) |
(Darker, 1932; Millar, 1984; Millar & Minter, 1980, Beenken, 2019) |
| (B). Ascomata (hysterothecia) | |||||
| Size (mm) | 0.6–2. 75 × 0.3–0.63 | 300–2,500 × 250–550 | 1–6 (22) | 0.5–2 × 0.4–0.8 | (4.5−) 13–22 × 0.28–0.4 |
| Depth (µm) | ca 280 | – | 350–370 | – | 180–280 |
| Opening | longitudinal split along stomata | Longitudinal split | – | longitudinal split along stomata | Vertical row of cells |
| Paraphyses | |||||
| Size (µm) | 180- 200 × 1–3 | 2.5–3.5 (width) | 120–140 × 2–3.5 | 2–3 (width) | (80−) 90–120 × 1.5–2.0 |
| Gelatinous sheath/Mucous coat | Present (inconspicuous) | Present | – | Present | Present |
| Septation | Present | Present | Present | Present | Present |
| Asci | |||||
| Size (µm) | 160–225 × 17–21 | 55–80 × 8–13 | 95–162 | 110–150 × 14–18 | (70−) 85–110 (−120) - ×14–18 (−20) |
| Opening mechanism | No obvious pre-formed apical apparatus (small apical hole or split after spores are released) | Opening by a large apical hole | – | No obvious pre-formed apical apparatus (small apical hole or split after spores are released) | Unitunicate |
| Number of spores | 8 | 8 | 8 | 8 | 8 |
| Ascospore | |||||
| Size (µm) | 68- 78 × 3–3.5 | 30- 50 × 2.5–3.5 | 23–53 × 2.5–3.5 | 30–65 × 2.5–3.5 | 22–32 × 6–7.5 |
| Mucilaginous/gelatinous sheath | Present | Present | Present | Present | Lacking |
| Hosts (number of needles) | Pinus contorta (2), P. elliottii var. elliottii (3), P. ponderosa (3), P. taeda (3), P. sylvestris (2) | Pinus ayacahuite (5) | Pinus ponderosa (3), P. attenuata (3) | Pinus kesiya (3, sometimes 2 or 4) | Pinus strobus (5) |
| Distribution | Western USA | Mexico | Western USA | Asia | Canada, USA |
| Conidiomata | Not observed (present in P. contorta) | Not observed | Present | Only fresh collected specimens | Not reported |
| References | (Darker, 1932; Millar, 1984; Minter & Millar, 1993d) | (Minter, 1988b) | (Staley & Bynum, 1972) | (Minter, 1993) | (Corlett & Shoemaker, 1984; Merrill, Wenner & Dreisbach, 1996) |
DNA extraction and sequencing
Cultures from single-spore isolations of L. montivaga, L. concolor and L. arcuata were attempted but did not yield pure cultures, as these are thought to be potentially obligate fungi. Similar to previous observations (Darker, 1932), mature spores isolated did not germinate and development of germ tubes in a few spores became arrested. Therefore, to be able to extract adequate amounts of quality DNA, fruiting bodies from three to five symptomatic needles from each tree were used for DNA extraction. DNA was extracted using a CTAB method with slight modifications in tissue grinding (Cubero et al., 1999). To prepare the samples, hysterothecia were cut into one mm long pieces and placed in two mL centrifuge tubes with one five mm glass bead and two 2.3 mm metal beads. To grind the samples, the tubes were submerged in liquid nitrogen before grinding using FastPrep (MP Biomedicals) for 30 s at speed 4 or 5. This previous process was repeated three times prior to the CTAB DNA extraction procedure developed by Cubero et al. (1999). DNA quantification and purity were assessed using NanoDrop 1000 Spectrophotometer (Thermo Scientific). Meanwhile, the DNA extraction of L. sulcigena and L. conjuncta samples was performed in Europe. Single fruiting bodies (ca. 3–4 mm long pieces) were prepared out of dry pine needles. DNA was extracted from the lyophilized and ground fruit bodies using the KingFisher/Flex Purification System (ThermoFisher Scientific) according to the manufacturer’s protocol and the chemicals for automated DNA extraction from fungal samples with Kingfisher 96/Flex supplied by LGC Genomics GmbH (Berlin).
DNA was amplified at the following loci: internal transcribed spacer region 1, 5.8S ribosomal RNA and internal transcribed spacer region 2 (ITS), large subunit ribosomal nucleic acid (LSU), and translation elongation factor (TEF1α). Primers used include ITS1 and ITS4 (White et al., 1990), LROR and LR5 or LR6 (Vilgalys & Hester, 1990), and EF1-983F and EFgr (Rehner, 2001). The ITS locus was amplified at optimal annealing temperatures between 50–55 °C with 30 cycles while TEF1α and LSU were amplified at 56° C annealing temperature with 35 cycles and other cycle parameters following Tanney & Seifert (2017). Amplification of each locus was performed in a 25-µL PCR reaction mixture of 1 × standard Taq reaction buffer, 0.2 mM of each dNTP, 0.4 µM of forward and reverse primer set, 0.625 units Taq polymerase, and 40 ng template DNA. For ITS amplification, the cycle parameters included initial denaturation at 94 °C for 2 mins, followed by 30 cycles of denaturation at 94 °C for 40 s, optimal annealing temperature for 40 s, extension at 72 °C for 1 min, and final extension at 72 °C for 5 mins.
PCR products were purified using ExoSAP-IT (Affymetrix™). All purified amplicons were sent to Eurofins Genomics LLC for sequencing. Additionally, cloning of PCR products for each locus was performed on at least three randomly selected L. concolor and L. montivaga samples using pGEM® T-Easy Vector Systems (Promega) to confirm that a sequenced amplicon was of single species. Three to seven clones were sequenced for each locus per sample and found to be 99.81 to 100% identical to the sequence of its corresponding original PCR product. Sequences were compared to NCBI sequence database using the Nucleotide Basic Local Assignment Search Tool (BLASTn) and were accessioned in NCBI GenBank (Table 1). Sequence data were trimmed and manually checked using Geneious version R9.0.5 (Biomatters, Auckland, New Zealand) and subsequently aligned using MUSCLE (Edgar, 2004). Polymorphic sites were determined using DnaSP (Rozas et al., 2003).
Phylogenetic analyses for each locus were constructed using Bayesian inference (MrBayes; Huelsenbeck & Ronquist, 2001) and maximum likelihood methods (PhyML; Guindon et al., 2010) as modules in Geneious v. R9.0.5. Optimal substitution models for each dataset generated using DT-ModSel (Minin et al., 2003) were as follows: SYM + G for ITS, TrNef + G for TEF1 α, TrN + I + G for LSU, and SYM + I + G for the concatenated dataset. For models of evolution that are not available in either MrBayes or PhyML modules, the next best complex models were applied. Bayesian tree was analyzed by running Markov Chain Monte Carlo (MCMC) for up to 1,100,000 generations with four heated chains. Maximum likelihood tree was analyzed using 1,000 bootstraps. Bayesian and maximum likelihood trees were generated with support thresholds of 80% with a 20% burn-in and 50%, respectively. The phylogenies were rooted to Chalara spp. (Chalara sp. MFLU 18-1812 and Chalara sp. MFLU 15-3167) following Ekanayaka et al. (2019).
To evaluate the congruence of the three loci dataset, partition homogeneity test was conducted using PAUP version 4.0a (Barker & Lutzoni, 2002). This resulted in a p-value =0.99, indicating congruence among the ITS, LSU and TEF1α datasets. Tree topologies from individual loci were also compared using the reciprocal 70% bootstrap approach (Mason-Gamer & Kellogg, 1996). Similarly, results also revealed no significant incongruence between the three datasets. Thus, the three loci dataset was combined using Sequence Matrix (Vaidya, Lohman & Meier, 2011). The alignment and consensus tree of the concatenated dataset were stored in TreeBase (Submission ID 26836). Published sequences of known related species in GenBank database were included in the phylogenetic analysis (Table S1). The rhytismataceous species were selected based on similarity to Lophodermella sequences and availability in NCBI database.
| No. | Character | Character States |
|---|---|---|
| (A). Characters to assess genus delineation | ||
| 1 | Ascomata: Shape | 0 non-linear or -elliptical, 1 mostly linear, nervisequious, dark brown to black, 2 mostly elliptical to elongate, concolorous to black |
| 2 | Ascomata: Position on substrate/host tissue (median transverse section) | 0 external/superficial, 1 subcuticular, 2 intraepidermal, 3 subepidermal, 4 subhypodermal |
| 3 | Asci: Shape | 0 more or less broadly saccate to clavate, 1 narrowly clavate or cylindrical |
| 4 | Ascospores: Shape | 0 acicular, 1 filiform, 2 clavate, 3 cylindrical, 4 fusiform to oval, 5 rod-shaped, 6 double spindle-shaped, 7 ellipsoid to fusiod |
| 5 | Ecological character: Host | 0 non-pine, 1 pine |
| (B). Characters to assess species delineation | ||
| 1 | Ascomata: length | 0 hysterothecia ≥ 1 mm, 1 hysterothecia short |
| 2 | Ascomata: color | 0 brown, 1 concolorous |
| 3 | Ascomata: fusion | 0 not fused, 1 fused |
| 4 | Ascospores: shape, size | 0 short (23–60 µm) clavate, 1 elongate clavate (68–90 µm), 2 fusiform to oval, 3 cylindrical, 4 ellipsoid to fusoid |
| 5 | Asci: number of spores | 4 four-spored, 8 eight-spored |
| 6 | Host: number of pine needles | 2 two-needle pine, 3 three-needle pine, 5 five-needle pine |
Character mapping
Morphological characters were selected based on the presence in literature and their use for taxonomic classification of Rhytismataceae. Characters were coded based on published descriptions (Table 3; Darker, 1932; Darker, 1967; Minter, 1988a; Minter & Millar, 1993a; Minter & Millar, 1993b; Minter & Millar, 1993c; Tanney & Seifert, 2017, MycoBank Database, 2016; Fungi and Lichens of Great Britain and Ireland, 2019) and then mapped on the Bayesian ITS dataset phylogeny which had a more comprehensive set of Rhytismataceae species in well-supported clades. To assess distinct morphological characters among Lophodermella species, key characters were selected based on Darker (1932) and Hunt & Ziller (1978). These were then mapped on a separate Bayesian ITS phylogeny (GTR+I+G model) that was limited to Lophodermella species and two outgroups (Elytroderma deformans and Chalara sp.). All morphological characters were coded as unordered and mapped with parsimony ancestral trace reconstruction using Mesquite v.3.6 (Maddison & Maddison, 2018).
Results
Molecular and phylogenetic analyses
PCR amplification produced a single band for each sample per locus. Chromatograms for forward and reverse sequences did not show multiple peaks at base calls, indicating uniform amplicons. Amplicons of the ITS, TEF1α and LSU yielded products that ranged from 347 to 543, 678 to 811 and 790 to 1,077 base pairs, respectively. Of the 40 samples of Lophodermella spp. and L. dooksii at the ITS, a total of nine genotypes were found with 83 polymorphic (segregating) sites and 64 parsimony informative sites were observed. At the TEF1 α, the 37 samples of Lophodermella species and L. dooksii had eight genotypes, and 77 of the 105 polymorphic sites were considered informative. Sequences of the 35 Lophodermella spp and L. dooksii samples at the LSU resulted in nine genotypes with 106 total polymorphic sites and 62 parsimony informative sites. BLAST results of sequences are presented in Table S2 .
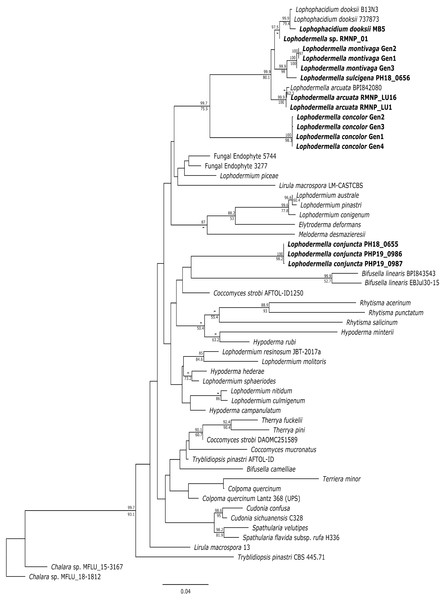
Several Lophodermella spp. and L. dooksii clustered in a well-supported clade (hereinafter referred to as the LOD clade) at the ITS, LSU and TEF1α phylogenies. This clade composed of genotypes of L. montivaga, L. concolor, L. arcuata, L. sulcigena, Lophodermella sp. and L. dooksii in the ITS phylogeny was well-supported in the Bayesian phylogeny with a 0.96 posterior probability (PP), excluding L. conjuncta (Fig. S1). Similarly, for the LSU phylogeny, both Bayesian and ML phylogenies produced the same clade well-supported clade (1.0 PP and 97.9 bootstrap support (BS); Fig. S2). Lophodermella conjuncta remained distinct from the clade representing all other Lophodermella species at the LSU phylogeny. At the TEF1α region, LOD clade had high support at 1.0 PP and 94.4 BS, (Fig. S3), but did not include both L. concolor and L. conjuncta. Similar to the ITS and LSU phylogenies, the concatenated phylogeny showed all Lophodermella species, except L. conjuncta, that were sampled in this study, as well as L. dooksii, belonged to a well-supported clade with 0.99 PP and 75.5 BS support values (Fig. 2). Distance matrix is shown in Table S3.
Figure 2: Maximum likelihood phylogeny depicting phylogenetic relationships of Lophodermella species within Rhytismataceae based on three gene regions including the internal transcribed spacer (ITS), large ribosomal subunit (LSU) and translation elongation factor 1-alpha (TEF1a).
Bayesian posterior probabilities (PP) greater than 0.80 and bootstrap (BS) support values from maximum likelihood analysis greater than 50 are shown above and below node, respectively. Species in bold are samples derived from this study. Numbers correspond to genotypes after concatenation.Morphology and Phylogeny of Lophodermella on P. flexilis
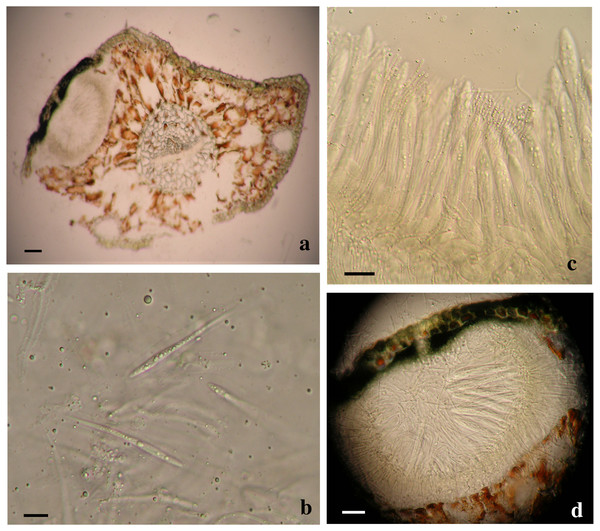
Based on the phylogenetic analyses, two separate Lophodermella species were collected from P. flexilis in the Rocky Mountain Region. Using the concatenated dataset, L. arcuata from Rocky Mountain National Park (RMNP_LU1 and RMNP_LU16) clustered with L. arcuata AY465518.1 from NCBI GenBank with 1.0 PP and 100 BS, whereas RMNP_01 clustered with Lophophacidium dooksii samples with 0.98 PP (Fig. 1). Similarly, RMNP_01 and L. dooksii (MB5) were found into a cluster with 0.98 PP and 89.9 BS, and 0.96 PP and 71.2 BS at the ITS (Fig. S1) and TEF1α (Fig. S3) trees respectively, indicating that RMNP_01 may represent a new species, distinct from L. arcuata. Morphologically, sample RMNP_01 had subhypodermal hysterothecia measuring 0.48–0. 6 × 0.16–0.168 mm and were tanned at mesophyll and hypodermis. Asci were broadly saccate measuring 96–130 × 12–14 µm. Ascospores were clavate, measuring 58–76 µm long and 3.8–4 µm wide. Ascospores were also covered with mucilaginous sheath (10 µm wide, Fig. 3). These fit the morphometric traits of L. arcuata (Table 2). Further, both Lophodermella sp. and L. arcuata were found on P. flexilis in similar geographic location.
Figure 3: Morphological characters of Lophodermella. sp. on Pinus flexilis collected from Rocky Mountain National Park, Colorado, USA.
Subhypodermal hysterothecia with tanned mesophyll and hypodermis (A, B), clavate ascospores with gelatinous sheath (B) and broadly saccate asci (C). Size bars A, C and D 20 mm; B 10 mm.Shared characteristics of Lophodermella clade
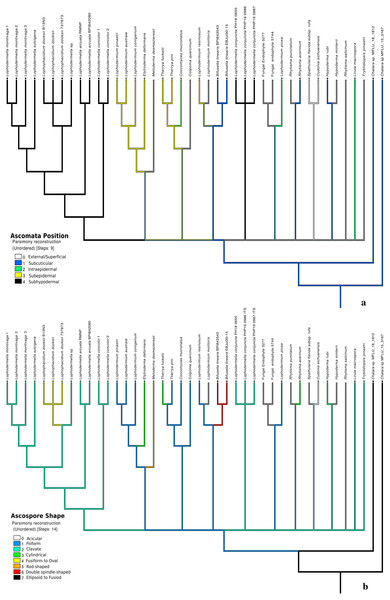
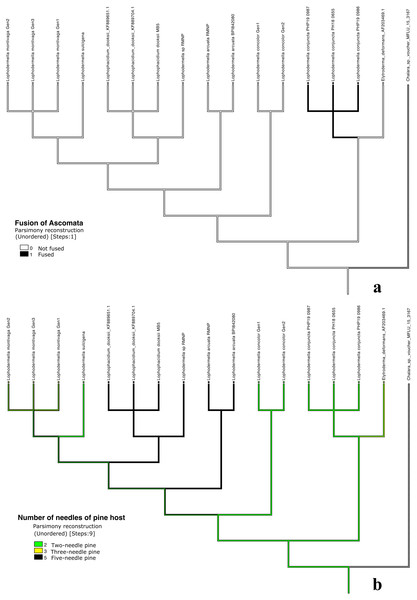
Five traits were used in this study due to the unavailability of morphological data or unclear morphological distinctions of other species within Rhytismataceae (Table 3, Table S4). The first four morphological characteristics included were those described by Darker (1967) as key characteristics of species within Lophodermella. These included ascomata shape and position, asci shape and ascospore shape. Host was included as an ecological trait. The only character conserved within the LOD clade composed of the five Lophodermella species and L. dooksii was subhypodermal ascomata position in a median transverse section (Fig. 4). All of the Lophodermella species sampled in this study occur on pine hosts. The shape of ascomata or hysterothecia, asci and ascospores differed within the LOD clade. Lophodermella hysterothecia were mostly elliptical and elongated while hysterothecia of Lophophacidium dooksii were linear. Lophodermella had clavate ascospores while ascospores of L. dooksii were fusiform to oval. All species in the clade, except L. concolor, had broadly saccate to clavate asci. To measure homoplasy and fit of characters, individual consistency (CI) and retention indices (RI) were measured. While all morphological characters obtained an RI ≥ 0.50, only ascomata position and ascospore shape had CI ≥ 0.50, which may imply synapomorphy of the two characters (Fig. 4).
Figure 4: Morphological characters mapped onto ITS phylogenetic tree with the parsimony ancestral reconstruction method using Mesquite v.3.6 with retention indices ≥0.50, ascomata position (A) and ascospore shape (B).
Distinct characters were observed across Lophodermella spp., which may be useful for species identification and delineation (Fig. 5, Table S5). Short and concolorous hysterothecia were distinct in L. concolor while elongated clavate ascospore and fused hysterothecia were distinct in L. conjuncta. The fusiform to oval ascospore was unique to Lophophacidium dooksii. Meanwhile, L. montivaga, Lophodermella sp. (RMNP_01) and L. sulcigena only differed at their host occurrence. Hysterothecia of L. arcuata was reported to be concolorous when dry as opposed to that of Lophodermella sp. (RMNP_01) which remains dark brown. All of the six characters for species delineation generated a mean CI and RI of 0.95 and 0.92, respectively.
Figure 5: Morphological characters mapped onto Bayesian ITS phylogenetic tree with the parsimony ancestral reconstruction method using Mesquite v.3.6.
Fusion of ascomata (A) and number of needles of pine host (B).Discussion
This study revealed a well-supported clade consisting of several Lophodermella species including L. montivaga, L. concolor, L. arcuata, L. sulcigena, and Lophodermella sp. within Rhytismataceae. Lophodermella conjuncta, however, was consistently placed outside of this clade. In all phylogenies, Lophophacidium dooksii consistently clustered within the LOD clade. Despite highly similar morphological characteristics, this study showed that Lophodermella pathogens are molecularly distinct from each other and may represent more genetic diversity than previously thought. This study also identified shared characteristics within the LOD clade and explored on morphological characters that could be useful in taxon classification.
Molecular and phylogenetic analyses of Lophodermella
A concatenated dataset of the three loci clearly separated L. montivaga and L. concolor that both infect P. contorta and distinguished the Lophodermella species from other closely related fungi. Lophodermella montivaga, L. concolor, L. arcuata, L. sulcigena, Lophodermella sp. and Lophophacidium dooksii formed the LOD clade, which were distinct from species within the genera Lophodermium (Ortiz-Garcia et al., 2003) and Spathularia-Cudonia (Ge et al., 2014). However, in the TEF1α phylogeny, L. concolor was excluded from the LOD clade, but was placed in the clade at the LSU and ITS phylogenies. This could be attributed to a fewer number of sequenced Rhytismataceae species resulting in low phylogenetic resolution or other genetic loci may best represent the species phylogeny. While additional sequences at each locus would likely improve phylogenetic resolution, whole-genome sequencing would provide greater advantage in phylogenetic reconstruction as well as gain deeper evolutionary perspectives on rhytismataceous needle pathogens.
Exclusion of L. conjuncta in the LOD clade may suggest polyphyly of the genus. This is the first report of the potential polyphyly of Lophodermella within Rhytismataceae. Polyphyletic genera are commonly observed within Rhytismatales partly due to the use of distinctive yet non-synapomorphic characters for generic-level classification (Lantz et al., 2011). Lophodermium is an example of a polyphyletic genus that appears in the radiate, bilateral and Picea-associated clades (2011). Reorganization of Lophodermium was not possible due to the wide diversity of species in the group (Darker, 1967). Monophyletic genera also exist within Rhytismataceae that includes Cudonia and Terriera (Lantz et al., 2011). However, this present study does not disregard potential changes in the phylogenetic arrangement and polyphyly as more Lophodermella species will be genetically investigated. Increased sampling of species within the two genera provided further evidence of Cudonia as a monophyletic genus but suggested that Spathularia was polyphyletic (Ge et al., 2014). It may also be possible that L. conjuncta belong to a separate genus that shares close morphological and phylogenetic relationship with Lophodermella. Thus, further investigation of other Lophodermella species which so far have no available sequence data still needs to be conducted to confirm these phylogenetic arrangements.
The present study supported a close relationship of L. montivaga and L. sulcigena compared to the other species within the LOD clade. Darker (1932) speculated that L. sulcigena from Europe may be identical to L. montivaga due to morphological similarities. Despite the overlapping morphological distinctions between the two species, this present study provided molecular evidence that L. montivaga and L. sulcigena are two distinct species. Another previous speculation was the possibility that L. arcuata is a variety of either L. montivaga or L. sulcigena owing to its resemblance to both species and its limited occurrence (Darker, 1932). However, symptom and ascocarp development in both species were different and thus were maintained as two different species (Millar, 1984). Genetic evidence gave support that L. arcuata is distinct from L. sulcigena and L. montivaga.
Consistent nesting of Lophophacidium dooksii in a Lophodermella clade was observed in all phylogenies, which concurs with a previous molecular study (Laflamme et al., 2015). Results herein showed that L. dooksii is more closely related to Lophodermella sp. (from P. flexilis) than to L. montivaga and L. arcuata, and provides more evidence for the transfer of the species from Phacidiaceae to Rhytismataceae as proposed by Ekanayaka et al. (2019). We did not attempt to reclassify the taxon to Lophodermella since we did not have large sample size and type specimen to conduct further validations. Interestingly, L. dooksii was synonymous to Canavirgella banfieldii, a species classified under Rhytismataceae, but the former taxonomic name was given priority due to its earlier publication (Laflamme et al., 2015). In other studies, use of multiple loci supported the placement of Cudonia and Spathularia from Geoglossaceae to Rhytismataceae (Gernandt et al., 2001; Lantz et al., 2011; Ge et al., 2014), which these results also support (Figs. S1–S3).
Phylogeny of Lophodermella sp. from P. flexilis
Individual phylogenies in this study could not confirm the species identity of the Lophodermella sp. from P. flexilis collected at RMNP as it did not cluster together with L. arcuata samples. Aside from morphometric features, initial examination identified RMNP_01 sample as L. arcuata due to its occurrence on P. flexilis in Colorado. Minter & Millar (1993a), Minter & Millar (1993b); considered host preference and geographic distribution as criteria for identification of L. arcuata due to the consistent reports on this species being the only member of the genus occurring on five-needle pines in North America. However, genetic data suggests Lophodermella sp. may represent a separate species distinct from L. arcuata. Since needle samples with this potentially new species were only collected from one tree, we did not attempt to formally name the species but temporarily named at the genus level as Lophodermella. Further investigation needs to be conducted to differentiate this species with other Lophodermella species described in literature and to define the population diversity of L. arcuata. Further, results from this study also suggest that undescribed cryptic Lophodermella species exist on pine hosts.
Morphological and Lifestyle Traits of the Lophodermella clade
Classification of Rhytismataceae genera has been challenged by the limited morphological features for characterization. Darker (1967) revised the genera within the previous Hypodermataceae based on the characteristics of their ascomata or hysterothecia, asci, and pycnidia or a combination of these characters. Spore shape, septation and color were secondary characters to delimit the genera (Darker, 1967). Further, Lantz et al. (2011) described ascomata and spores as unreliable characters for genus delimitation in Rhytismatales but found that a combination with other traits was potentially useful. This study showed that, at the genus level, subhypodermal ascomata and ascospore shape may be used as diagnostic characters for delimitation of genus Lophodermella. This is congruent to the dichotomous key produced by Darker (1967) to delimit this genus. Despite its inclusion in the LOD clade, Lophophacidium dooksii did not have clavate ascospores but rather had ascospores with fusiform to oval shape. Interestingly, aside from subhypodermal hysterothecia, all species within the LOD clade produced a tanned hypodermis. Furthermore, despite low consistency, the strong retention of asci shape may also suggest its role in taxa distinction.
Within Lophodermella genus, morphometric traits such as size of ascospores and hysterothecia are still used as distinctive characters. This study showed that a combination of morphological and ecological characters may be used to distinguish Lophodermella species, particularly ascospore and hysterothecia length, hysterothecia color, and the number of needles on pine host. However, these characters may also become problematic in practice. For example, while ascospore size was identified as a reliable criterion, measurements of spores varied depending on the freshness of specimen and thus cannot easily be used for identification of Lophodermella species (Millar, 1984). Further, concolorous hysterothecia as key character may be misleading as some species can also produce conspicuous hysterothecia (Millar, 1984).
Difficulty in obtaining pure cultures of L. montivaga, L. concolor and L. dooksii can also potentially limit further characterization of other traits such as physiology and pathogenicity. Similar to other studies, we were not able to grow in culture the Lophodermella species sampled in this study, suggesting an obligate lifestyle. Use of agar cultures including pine extract agar did not yield successful cultures of Lophodermella (Millar, 1984). Some studies also described L. dooksii and Bifusella linearis as obligate fungal pathogens after unsuccessful attempts of obtaining cultures or only obtaining short-lived cultures (Broders et al., 2015; Merrill, Wenner & Dreisbach, 1996). In contrast, previous studies were able to isolate pure cultures of L. sulcigena on malt agar (Jalkanen, 1985; Kowalski & Krygier, 1996). Similarly, a number of studies documented several Lophodermium species (e.g., Decker, Hsiang & Peterson, 2001; Wilson et al., 1994) growing in 2% malt extract agar. Elytroderma deformans needed an acidic pine decoction agar substrate or an addition of pine needle extracts to significantly grow in culture (Laurent, 1962; Legge, 1964). Consequently, while environmental DNA may be available, the absence of pure cultures of many Lophodermella species limit further molecular research that require a high pure DNA concentration.
Most Lophodermella species appear to be either specific to a single host species distributed in a certain geographic region (i.e., L. maureri on P. ayacahuite in Mexico and L. orientalis on P. kesiya in Asia) or to a group of host species within a Pinus classification with similar number of needles (i.e., L. sulcigena and L. conjuncta on two-needle pines of subsection Pinus in Europe, and L. concolor on two-needle pines of subgenus Pinus in western North America; (Millar, 1984; Gernandt et al., 2005). Furthermore, L. arcuata and L. maureri are the only two Lophodermella species on five-needle pines of subsection Strobus while L. morbida only occurs exclusively on three-needle pines under section Trifoliae. In contrast, L. cerina was reported to have a broader host range occurring on two- to three-needle Pinus species in sections Trifoliae and Pinus (subgenus Pinus; (Millar, 1984; Gernandt et al., 2005). Lophodermella montivaga was also documented on two- to five-needle Haploxylon and Diploxylon pines. In this study, genetic information was used to verify the association of Lophodermella species with a known host. It allowed us to identify additional species on P. flexilis that would have otherwise been classified as L. arcuata based on its morphology and host association. Thus, it can serve as a tool to assess the extent of these fungal species across different hosts in different geographic regions.
Conclusion
This study sequenced and characterized emerging Lophodermella needle cast pathogens on Pinus in North America and Europe. It highlights a distinct clade composed of Lophodermella species and Lophophacidium dooksii within Rhytismataceae. Further, this study also observed a Lophodermella species on P. flexilis that is morphologically similar yet genetically distinct from L. arcuata, which suggests presence of undescribed cryptic Lophodermella species. Further investigations of Lophodermella species using advanced molecular tools can also help answer genetic, evolutionary and ecological inquiries such as on population structure, pathogenicity, host specialization, hybridization, and other biological inferences.
Supplemental Information
Sequences downloaded from NCBI GenBank and used in phylogenies
Identity and the range of similarity and e-value of ≥50% of the sequences of each Lophodermella spp. and Lophophacidium dooksii generated from this study at the three loci as inferred from NCBI Basic Local Alignment Search Tool (BLAST).
Maximum likelihood distance of Lophodermella spp. with Lophodermium spp. and Elytroderma deformans in the concatenated dataset
Morphological characters and character states of the Lophodermella and non-Lophodermella species
Morphological characters and character states of Lophodermella species, including Elytroderma deformans and Chalara sp. as outgroups
Maximum likelihood phylogeny depicting phylogenetic relationships of Lophodermella montivaga and L. concolor within the Lophodermella clade based at the internal transcribed spacer (ITS)
Bayesian posterior probabilities (PP) greater than 0.80 and bootstrap (BS) support values from maximum likelihood analysis greater than 50 are shown above and below node, respectively. Species in bold are samples derived from this study. Lophodermella concolor and L. montivaga are distinguished per site within Gunnison National Park, CO, USA: CS –Cold Springs, FS –Fisherman Trail, LP –Lodgepole Campground, LV –Lakeview Campground, OJ –Oh Be Joyful, PT –Pitkin, SR –Slate River, TC –Tincup, TL - Taylor. Other Lophodermella species: RMNP –Rocky Mountain National Park, Colorado. Numbers correspond to genotype.
Maximum likelihood phylogeny depicting phylogenetic relationships of Lophodermella montivaga and L. concolor within the Lophodermella clade based at the large ribosomal subunit (LSU)
Bayesian posterior probabilities (PP) greater than 0.80 and bootstrap (BS) support values from maximum likelihood analysis greater than 50 are shown above and below node, respectively. Species in bold are samples derived from this study. Lophodermella concolor and L. montivaga are distinguished per site within Gunnison National Park, CO, USA: CS –Cold Springs, FS –Fisherman Trail, LP –Lodgepole Campground, LV –Lakeview Campground, OJ –Oh Be Joyful, PT –Pitkin, SR –Slate River, TC –Tincup, TL - Taylor. Other Lophodermella species: RMNP –Rocky Mountain National Park, Colorado, AT –Austria and CH –Switzerland. Numbers correspond to genotype.
Bayesian phylogeny depicting phylogenetic relationships of Lophodermella montivaga and L. concolor within the Lophodermella clade based at the translation elongation factor 1-alpha (TEF1a)
Bayesian posterior probabilities (PP) greater than 0.80 and bootstrap (BS) support values from maximum likelihood analysis greater than 50 are shown above and below node, respectively. Species in bold are samples derived from this study. Lophodermella concolor and L. montivaga are distinguished per site within Gunnison National Park, CO, USA: CS –Cold Springs, FS –Fisherman Trail, LP –Lodgepole Campground, LV –Lakeview Campground, OJ –Oh Be Joyful, PT –Pitkin, SR –Slate River, TC –Tincup, TL - Taylor. Other Lophodermella species: RMNP –Rocky Mountain National Park, Colorado, AT –Austria and CH –Switzerland. Numbers correspond to genotype.