Abstract
Chronic prurigo (CPG) is a neuroinflammatory, fibrotic dermatosis that is defined by the presence of chronic pruritus (itch lasting longer than 6 weeks), scratch-associated pruriginous skin lesions and history of repeated scratching. Patients with CPG experience a significant psychological burden and a notable impairment in their quality of life. Chronic prurigo of nodular type (CNPG; synonym: prurigo nodularis) represents the most common subtype of CPG. As CNPG is representative for all CPG subtypes, we refer in this review to both CNPG and CPG. We provide an overview of the clinical characteristics and assessment of CPG, the burden of disease and the underlying pathophysiology including associated therapeutic targets. The information provided results from a PubMed search for the latest publications and a database search for current clinical trials (ClinicalTrials.gov, EU Clinical Trials Register [European Medicines Agency]; using the following terms or combinations of terms: ‘chronic prurigo’, ‘prurigo’, ‘prurigo nodularis’, ‘pathophysiology’, ‘therapy’, ‘biologics’, ‘treatment’). Dupilumab is the first authorized systemic therapy by the European Medicines Agency (EMA) and the US Food and Drug Administration (FDA) for CNPG to date. Topical and systemic agents that are currently under investigation in clinical randomized, placebo-controlled phase II and III trials such as biologics (e.g., nemolizumab, vixarelimab/KPL-716, barzolvolimab/CDX-0159), small molecules (ruxolitinib cream, povorcitinib/INCB054707, abrocitinib) and the opioid modulator nalbuphine are highlighted. In the last past 15 years, several milestones have been reached regarding the disease understanding of CPG such as first transcriptomic analysis, first terminology, first guideline, and first therapy approval in 2022, which contributed to improved medical care of affected patients. The broad range of identified targets, current case observations and initiated trials offers the possibility of more drug approvals in the near future.
Similar content being viewed by others
In the past 15 years, important cornerstones for the understanding of chronic prurigo were laid (e.g., first disease definition, first guideline, first clinical trials, first approved systemic therapy). |
The growing knowledge of the pathophysiology of chronic prurigo including new therapeutic targets enables the development of new, promising drugs that are urgently needed, as the burden of disease is high for affected patients. |
1 Introduction
Chronic prurigo (CPG) is a neuroinflammatory, fibrotic dermatosis and it is defined by the presence of chronic pruritus (itch lasting longer than 6 weeks), scratch-associated pruriginous skin lesions and history of repeated scratching [1]. Pruriginous lesions are clinically very typical and are composed of epidermal acanthosis, dermal fibrosis with a dense inflammatory infiltrate. The lesions usually persist for months or even years. Neuronal sensitization to itch and the subsequent development of an itch–scratch cycle have been described as the mechanism of origin [2]. Based on these aspects, CPG represents a distinct disease [1, 2]. This review aims to describe the characteristics and novel therapy of this disease.
2 Methods
We conducted a PubMed search of all publications since 1982 (531 results with the term ‘chronic prurigo’; 1680 results with the term ‘prurigo’ in August 2023) and a database search of current clinical trials (ClinicalTrials.gov, EU Clinical Trials Register [European Medicines Agency], using the following terms or combinations of terms: ‘chronic prurigo’, ‘prurigo’, ‘prurigo nodularis’, ‘pathophysiology’, ‘therapy’, ‘biologics’, ‘treatment’). A systematic literature review was performed to identify randomized controlled trials (RCTs) according to the Cochrane recommendations. As of August 2023, the following studies could be found with the term ‘prurigo’:
-
ClinicalTrials.gov: 34 results (22 of these were considered in this review).
-
EU Clinical Trials Register: 24 results (16 of these were considered in this review).
3 Terminology, Epidemiology and Clinics
The terminology for CPG has been imprecise and unclear since the first description of chronic nodular prurigo (CNPG, also termed prurigo nodularis [PN]) by Hyde in 1909 [3, 4]. Several sub-entities, either defined by the lesion type or the first describer, have been published. This is understandable as the composition of pruriginous lesions differ from patient to patient. The umbrella term of CPG takes this into account and makes it unnecessary to define a subtype [4]. Moreover, using an umbrella term in clinical trials is also good for patients as all can benefit from novel therapies, not just one subtype. Most patients have nodules; accordingly, the subtype CNPG was specifically considered in basic and clinical trials due to feasibility in recruitment. As CNPG is representative of all CPG subtypes, we refer in this review to both CNPG and CPG.
All age groups can be affected by CPG, although affected patients tend to be older [5, 6]. Various epidemiologic data on the prevalence of CNPG exist and show that it occurs worldwide. CNPG, with a prevalence of 0.21% in Germany in 2010 [7] and 58 per 100,000 overall in the United States in 2019 [8], is a relatively rare disease, though these numbers likely reflect an underestimation given poor disease awareness and many patients not seeking medical help [8]. CNPG has a disproportionate impact on African Americans, as evidenced by Black patients being diagnosed with CNPG at a rate 3.4 times higher than white patients [9]. Additionally, CNPG is linked to atopy in a subset of patients (33.1% of patients, as reported in a European cross-sectional study by Pereira et al. [6]). This fact sometimes leads to the assumption that CNPG is a subtype of atopic dermatitis (AD). However, CNPG can also develop apart from atopy and is not a variant of another dermatosis, which is also true for CNPG occurring in bullous pemphigoid or lichen planus. Moreover, important disease-specific differences between CNPG and AD have been shown recently using single-cell RNA sequencing [10, 11].
CNPG is characterized by a severe and persistent pruritus that surpasses the itch intensity, frequency and impact on quality of life observed in other chronic pruritic skin conditions like atopic dermatitis and psoriasis [12]. The intensity of pruritus is independent of the patient's age and sex and the possibly presence of atopy [6]. Thus, atopic diathesis does not seem to be a relevant factor for the intensity of pruritus in CNPG.
The pruriginous lesions of CPG may differ in the type of lesion, number, color (skin-colored, pink, or red) and the presence of excoriations. Pruriginous lesions can be divided into papules (< 1 cm in diameter), nodules (> 1 cm in diameter), plaques and umbilicated papules/nodules or linear lesions [1, 13]. Within a single individual, multiple types of lesions may coexist [1]. Depending on the dominant lesion type, CPG can be divided into subtypes, of which CNPG is the most common [1]. The subtypes show no differences in terms of intensity of pruritus, underlying origin and quality-of-life impairment, emphasizing that all subtypes can be assigned to the umbrella term ‘CPG’ [14].
Patients with CPG experience a significant psychological burden and a notable impairment in their quality of life [5, 7, 8, 15]. A comparison between patients with CPG and patients with chronic pruritus without visible skin lesions or scratch lesions revealed that the disease burden is not solely attributed to the presence of severe pruritus but to the presence of pruriginous lesions [16]. However, CPG patients consider the presence of itch as the most burdensome aspect of the disease [6]. In addition to experiencing higher intensities of pruritus, patients with CPG are more prone to reporting daily pruritus and to experiencing both daytime and nighttime pruritus, while being less likely to have spontaneous remission of pruritus compared with those with patients with chronic pruritus without visible skin lesions or scratch lesions [16]. Given these observations, it is not surprising that sleep disturbances and other psychological symptoms, including anxiety and depression, contribute significantly to the high disease burden experienced by patients with CPG [16]. A study involving 263 patients diagnosed with CPG demonstrated that an overwhelming majority of patients (97.2%) experienced sleep disturbances as a result of having pruritus [17]. The results showed that patients lost a median of 2 h of sleep per night due to itching [17]. These findings highlight the significant disruption that pruritus can cause in terms of sleep patterns, further emphasizing the multifaceted impact of chronic pruritus on patients' well-being.
Psychiatric diseases can be triggered by the presence of CPG or may preexist as comorbidities [18]. It is crucial to emphasize that CPG itself is not a psychiatric disorder, despite previous misconceptions. However, as a reaction to long-standing prurigo, increased rates of depression, greater usage of antidepressant medications and an inclination towards suicidal tendencies have been described in patients with CPG [19]. In particular, patients with CPG who engage in automatic scratching, even in the absence of itch, exhibit elevated levels of psychological impairment and heightened reactivity to stress [20]. This is in contrast to CPG patients who only scratch when itch is present, suggesting that automatic scratching behavior is associated with greater psychological and stress-related challenges. Overall, patients with CNPG have a three times higher risk for depression compared with patients with other inflammatory dermatoses [21]. Moreover, the severity of depression has been shown to have a direct impact on the intensity of pruritus, underscoring the importance of considering the patient's mental state in treatment approaches [22].
The financial burden associated with CPG has received limited evaluation. However, a European cross-sectional study involving 406 patients diagnosed with CNPG shed some light on this aspect. The study revealed that a majority of patients (59%) reported out-of-pocket costs amounting to 500 € or more within the previous 6 months. These costs encompassed expenses related to treatments, including personal purchases, prescribed medications and travel costs incurred for physician visits [17].
4 Pathophysiology of Chronic Prurigo and Therapeutic Targets
The pathophysiology of CPG is still not fully understood. However, recent transcriptomic studies, including one investigating DNA methylation, clearly revealed that CPG, and CNPG in particular, are separated from other entities (especially atopic dermatitis or psoriasis) by some unique features such as collagen and neural dysregulation [11, 23,24,25,26,27]. In addition, the analyses clearly separate pruritic lesional CNPG skin from non-pruritic CNPG skin and from healthy volunteers [27]. By showing this, they confirm previous histological observations that epidermal keratinocytes and nerve fibers, dermal vessels and fibroblasts are involved in the pathophysiology of CNPG. Increased expression of nerve growth factor (NGF), matrix metalloproteinases, CXCL2 and insulin-like growth factors, for example, in CNPG compared with AD point to complex mechanisms and multiple cross-interactions of different skin cell types [11]. Signatures of extracellular matrix organization, collagen synthesis and fibrosis have been identified [10]. Several of these have been confirmed already on a morphological level by immunostainings such as the extracellular matrix protein periostin [28]. Regarding fibroblasts, a unique population of CXCL14-IL24+ secretory-papillary fibroblasts as well as increased high fibroblast levels of neuromedin B have been identified [10]. Fibroblasts have also been the focus of another recent single-cell analysis. In this study, a cancer-associated fibroblast (CAF)-like phenotype (with WNT5A+ CAFs) has been shown to be increased in lesional CNPG skin [29].
The dermal inflammatory infiltrate of CNPG is composed of mainly T lymphocytes, but also increased numbers of 2D7+ basophils and major basic protein (MBP)+ eosinophils. These dermal cells express IL-4, IL-13 or IL-31 to a higher level as compared with non-lesional or healthy skin. Interestingly, IL-4 is expressed principally by CD3+ T lymphocytes and 2D7+ basophils. IL-13 is mostly expressed by MBP+ eosinophils [30]. IL-31 was found in T lymphocytes but also in CD11c+ dermal myeloid dendritic cells which have been shown to be abundantly present in lesional CNPG skin [31]. The itch intensity was shown to be associated with the level of IL-31 pathway protein and receptor expression [32]. Though this resembles the profile of atopic dermatitis, a recent single-cell RNA sequencing combined with T-cell receptor sequencing further revealed immune activation pathways in CNPG, interestingly to a lesser extent than in atopic dermatitis [10]. Moreover, differentially expressed genes point to involvement of Th1/Th17/Th22 signatures next to Th2 in the pathogenesis of CNPG [23].
Elevated immune signatures have also been found in the blood, including IL-13 and IL-31 [33,34,35,36,37]. Different ethnic clusters have been described [37]. To date, this is of uncertain relevance but might point to the importance of immune mechanisms in the pathophysiology.
In CNPG, reduced intraepidermal nerve fiber density was demonstrated in several studies [27, 38,39,40]. After healing of pruriginous lesions, nerve fiber density returns to normal [39]. A recent study further demonstrated a specific level of epidermal branching of single nerve fibers, possibly of reactive nature, assessed by a semiquantitative pattern analysis. Corresponding to this, axonal growth-promoting NGF genes were found to be upregulated in CNPG [27]. The altered epidermal neuroanatomy is associated with an altered nerve fiber function and neuronal hypersensitivity [27, 30, 41, 42]. In chronic pruritus of various origins, hyperknesis due to electrical stimulation was found, suggesting common central neuronal sensitization mechanisms [43]. In CNPG, stimulation with cowhage resulted in increased itch intensity in lesional CNPG skin compared with healthy controls, suggesting neuronal sensitization of mechano- and heat-sensitive C-fibers [41]. Further, another sign of neuronal sensitization is sensitivity to touch-evoked itch by pinprick stimuli (punctate hyperknesis), which is significantly higher at the site of pruriginous lesions than non-lesional skin in CNPG patients. According to Hashimoto et al., this might be due to the altered epidermal neural structures and the impact of the immune mechanisms [30]. Interestingly, pain-transmitting mechanisms remain unchanged in CNPG [38].
Several studies confirm that these mechanisms might be based on bridging the immune and peripheral nervous system. It is known, for example, that Th2 cytokines such as IL-4 or IL-13 can directly lead to hypersensitivity of nerve fibers. According to this, therapies in CNPG either target the immune system (such as antibodies against IL-4 or IL-31) or the nerve fiber system (such as nalbuphine; see Fig. 1).
Therapeutic targets for chronic nodular prurigo (CNPG). In CNPG therapy, different targets can be addressed to improve pruriginous lesions and chronic pruritus. The adaptive immune response and the peripheral nerve system provide many promising therapeutic targets for biologics, Janus kinase inhibitors and opioid modulators. IL interleukin, KOR κ-opioid receptor, MOR μ-opioid receptor
Still, the predisposition to CPG is unknown. A first genome-wide association analysis of patients in three different countries identified genetic variants associated with CNPG, including one near PLCB4 (gene encoding phospholipase C) and others near TXNRD1 (encoding thioredoxin reductase 1). Black patients have a > 2-fold greater genetic risk of developing CNPG [44].
5 Diagnosis
The diagnosis of CPG is made clinically. However, the origin of chronic pruritus needs to be identified. Accordingly, it is essential to obtain a comprehensive medical history, which includes itch-specific details (e.g., onset of itch, itch intensity) as well as a careful assessment of medications, comorbidities and the psychiatric history.
During the physical examination, the distribution and type of pruriginous lesions needs to be determined. Lesions can be single in number and localized to a single area or they can be numerous and spread throughout the entire skin [1]. The severity of CNPG is determined primarily by the number of lesions (see Table 1) and can be objectively assessed using validated clinician-reported outcome tools such as the Prurigo Activity and Severity (PAS) score [45] and Investigators Global Assessments (IGA) for chronic prurigo [46].
The physical examination also aims to identify associated skin diseases which might have induced pruritus and thus are an etiological factor in CNPG. For the same reason, the examination should include a check of mucous membranes and palpation of the liver, kidneys, spleen and lymph nodes. This can help identify any underlying systemic diseases contributing to CNPG [47].
To further investigate the potential underlying etiology of CNPG, a laboratory workup is recommended [47]. This may involve conducting a blood count, including a differential blood count, measuring C-reactive protein (CRP), evaluating liver function (AST, ALT, gGT, AP), assessing kidney function (creatinine, estimated glomerular filtration rate), measuring lactate dehydrogenase (LDH) and examining thyroid-stimulating hormone (TSH). Additional laboratory tests should be considered based on the patient's clinical history and the findings from the physical examination [47]. Radiological procedures such as lymph node and abdominal sonography, as well as chest radiography, are recommended to rule out malignancy as a potential cause of CNPG. This is particularly important for patients who have experienced pruritus for less than a year or state B symptoms (like fever, loss of more than 10 percent of body weight over a period of 6 months, drenching night sweats) [47].
Although CNPG is primarily diagnosed clinically, obtaining skin samples for histological examination and immunofluorescence can be beneficial in certain cases. This approach is especially useful when there is uncertainty about an underlying primary skin disease. In case reports, CNPG has been observed to mask conditions like bullous pemphigoid [48] or lichen planus [49]. In these cases, the preferred treatment approach combines antipruritic therapy with specific treatments targeting the underlying dermatoses. In order to be able to choose the necessary patient-specific diagnostics and work cost effectively, the patient should be evaluated in a specialized itch center [50].
5.1 Patient-Reported Outcome in Patients with CNPG
To accurately measure the intensity of pruritus in CNPG, it is necessary to use a reliable assessment tool that can evaluate the severity of pruritus [12]. The most frequently used instruments for assessing pruritus intensity are the numeric rating scale (NRS, range 0–10), visual analog scale (VAS, range 0–10) and verbal rating scale (VRS, range 0–4) [12]. To categorize the severity of pruritus, NRS and VAS commonly employ cut-off points of 3, 7 and 9. These points serve as thresholds to differentiate between mild, moderate, severe and very severe pruritus [51].
Inadequate control of CPG can significantly impact the patient's quality of life. To assess disease control from the patient's perspective, the Prurigo Control Test (PCT) was developed and validated. This questionnaire consists of five items that allow patients to evaluate and determine whether their CNPG is effectively managed or not. By answering these items, clinicians can assess the level of control of the CNPG from the patient's perspective (submitted; for further information see http://www.pruritussymposium.de/definition-of-severity-and-descriptors-of-chronic-prurigo-ndash-harmonization-of-european-prurigo-documentation.html).
To evaluate the consequences of CPG on individuals, standardized questionnaires can be utilized to assess the limitations in quality of life as well as the presence of depression and anxiety. Various instruments are available to measure quality of life, with the Dermatology Life Quality Index (DLQI) [52] and the pruritus-specific ItchyQoL [53] being the most commonly used ones for patients with CPG. Additionally, the Hospital Anxiety and Depression Score (HADS) [54] can serve as a screening tool to identify the presence of anxiety and depression in individuals with CPG.
5.2 Clinician-Reported Outcomes
The Prurigo Activity and Severity Score (PAS) [45] and the Investigator's Global Assessment (IGA) [46] were created to provide an objective and standardized way to document pruriginous lesions in all CPG subtypes. PAS captures information about the type, location and quantity of pruriginous lesions, including the percentage of active and healed lesions. The IGA stages comprise a simple rating system to evaluate the presence of pruriginous lesions based on four levels. The IGA also considers the scratch activity for the lesions. The classification of severity can be determined using the IGA or the PAS [46]. These assessment tools are not only utilized to evaluate and track the progress of CPG during treatment but also to establish inclusion criteria and therapy success in clinical trials.
6 Currently Available Therapies for CNPG
Although urgently needed, approved therapies and RCTs for CNPG are limited compared with other dermatological diseases like atopic dermatitis [55]. Dupilumab, a fully human monoclonal antibody targeting the interleukin (IL)-4 receptor α, represents the only approved systemic treatment (FDA, EMA) for adult patients with CNPG to date (June 2023) [56]. Other available pharmacological interventions that are recommended in the International Forum for the Study of Itch (IFSI) guideline on CPG (including CNPG), in addition to emollients, are off-label for CNPG [47].
Available therapeutic options for CNPG can be divided into the following groups:
-
(i)
Topical therapies.
-
▪
topical anti-inflammatory therapies (topical corticosteroids [TCS], intralesional corticosteroids, topical calcineurin inhibitors [TCI]: tacrolimus, pimecrolimus).
-
▪
topical analgesics (anesthetics, cryotherapy, the transient receptor potential vanilloid 1 [TRPV1] agonist capsaicin).
-
▪
topical antiproliferative agents (coal tar, vitamin D derivatives like calcipotriene).
-
(ii)
Ultraviolet (UV) phototherapy (e.g., narrowband ultraviolet B light [NBUVB], psoralen plus ultraviolet A (PUVA) and laser therapies (e.g., excimer laser).
-
(iii)
Systemic therapies.
-
▪
antihistamines.
-
▪
conventional immunosuppressants (oral corticosteroids, cyclosporine, methotrexate, azathioprine, thalidomide/lenalidomide).
-
▪
neuromodulating therapies (opioid modulators like naloxone, naltrexone and nalbuphine, neurokinin-1 (NK1) receptor (NK1R) antagonists like aprepitant and serlopitant, antidepressants like paroxetine, fluvoxamine and duloxetine, gabapentinoids).
-
▪
immunomodulating therapies (biologics like dupilumab, nemolizumab and oral Janus kinase inhibitors (JAKi) [47, 55].
The current IFSI guideline on chronic prurigo recommends a step-by-step treatment approach (step 1: TCS, TCI and H1 antihistamines; step 2: topical capsaicin, intralesional corticosteroids and UV therapy; step 3: gabapentin, pregabalin or antidepressants in case of predominating neuropathic characteristics versus cyclosporine or methotrexate in case of predominant inflammation; step 4: NK1R antagonist, μ-opioid receptor antagonists, dupilumab, nemolizumab (currently under investigation), (thalidomide) [47]. The step-wise approach is based on the few clinical trials available. An RCT in 10 CNPG patients, in which betamethasone valerate, a frequently used TCS in CNPG, was compared with the vitamin D derivate calcipotriol, showed that betamethasone valerate was inferior to calcipotriol in reducing CNPG skin lesions (p < 0.05) [57].
Siepmann et al. conducted a phase II RCT, in which 30 CNPG patients were treated with either 1% pimecrolimus cream or 1% hydrocortisone cream. After a treatment duration of only 10 days, the pruritus intensity was significantly reduced in both patient groups (p < 0.001); there were no differences regarding the strength of pruritus reduction between both topicals (p = 0.394) [58].
Currently (August 2023), there are no RCTs on antihistamines, topical capsaicin, intralesional steroids, gabapentinoids, antidepressants, cyclosporine, methotrexate, or azathioprine for CNPG [47].
Herein, we give an update on novel therapies and emerging agents for CNPG that are currently being investigated in RCTs. In phase III: nemolizumab (anti-interleukin-31 receptor A [IL-31RA] antagonist) and ruxolitinib cream (topical JAKi); in phase II: vixarelimab/KPL-716 (anti-OSM beta receptor antagonist), INCB054707 (JAK 1 inhibitor) and abrocitinib (JAK1 inhibitor); and in phase I: CDX-0159/barzolvolimab (tyrosine kinase KIT receptor inhibitor) (see ClinicalTrials.gov, EudraCT clinicaltrialsregister.eu). Approved therapies and current or completed double-blind placebo-controlled studies or RCTs for CNPG are additionally presented in Table 2. Conventional therapeutic options for CNPG are well described elsewhere and therefore are not described more in detail in this review [4, 47, 55, 59, 60].
6.1 Dupilumab: Efficacy and Safety of the First Approved Biologic for CNPG
The monoclonal antibody dupilumab interferes with type 2 helper T-cell (Th2)-mediated inflammation by inhibiting the shared α subunit for IL-4 and IL-13 of the IL-4 receptor [56].
It was first approved in March 2017 for adult patients with moderate-to-severe atopic dermatitis (AD), insufficiently controlled with topical therapies [61]. In Europe, dupilumab is approved for moderate-to-severe AD (patients aged ≥ 12 years), severe AD (patients aged ≥ 6 months), severe asthma (patients aged ≥ 6 years; add-on therapy), chronic rhinosinusitis with nasal polyps (adult patients; add-on therapy), eosinophilic esophagitis (patients aged ≥ 12 years) and moderate-to-severe CNPG (adult patients) [62].
Dupilumab was investigated in 151 and 160 adult patients, respectively, in the two double-blind, placebo-controlled phase III trials LIBERTY-PN PRIME and PRIME2 [56]. The patients received dupilumab 300 mg (loading dose 600 mg) subcutaneously (SC) or placebo every 2 weeks (Q2W) for 24 weeks and were allowed to continue stable therapies with low-to-moderate potency TCS or TCI during the trial. In the dupilumab arm, 37.2% of patients at week 12 (PRIME2) and 60.0% of patients at week 24 (PRIME) reached the primary endpoint of an improvement of Peak Pruritus Numeric Rating Scale (PP-NRS) ≥ 4 points (PRIME2 placebo arm: 22.0% of patients; 95% confidence interval [CI] for the difference, 2.3–31.2; p = 0.022; PRIME placebo arm: 18.4% of patients; 95% CI for the difference, 27.8–57.7; p < 0.001) [56].
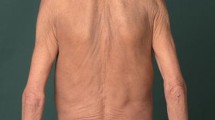
Dupilumab was also significantly superior to placebo regarding the improvement of skin lesions as measured by the Investigator Global Assessment for PN–Stage (IGA PN-S) [56]. A score of 0 (‘clear’) or 1 (‘almost clear’) was achieved by 48%/44.9% (PRIME/PRIME2) of patients at week 24 (placebo: 18.4%/15.9%; 95% CI for the difference, PRIME: 13.4–43.2; p < 0.001; PRIME2: 16.4–45.2; p < 0.001) as well as by 32.0%/25.6% of patients at week 12 (placebo: 11.8%/12.2%; 95% CI for the difference, PRIME: 7.8–34.0; p = 0.003; PRIME 2: 2.6–27.0; p = 0.019) [56]. Treatment-emergent adverse events (TEAEs) comprised conjunctivitis (PRIME/PRIME2: 2.7%/3.9% of patients) and herpes viral infections (PRIME2: 5.2% of patients). Skin infections (herpes infections excluded) occurred more often in placebo-treated patients [56]. Figure 2 demonstrates a CNPG patient who was successfully treated with dupilumab.
A few cases report good efficacy and safety of dupilumab in children and adolescents with CNPG (off-label use) [63,64,65].
Husein-ElAhmed and Steinhoff compared the efficacy of dupilumab in patients with AD and CNPG and concluded that the onset of action of dupilumab is faster regarding the decrease of pruritus in AD than in CPNG and patients with atopic CNPG may require longer treatment periods [66].
So far, long-term studies and large real-world data collections investigating efficacy, safety and potentially disease-modifying effects of dupilumab in CNPG are lacking.
7 Promising Therapeutic Approaches in Clinical Trials
The agents that are currently under investigation are either monoclonal antibodies (administered SC or intravenously) that target central itch signaling pathways by inhibiting for example the IL-31 receptor A/oncostatin M receptor β (like nemolizumab or vixarelimab) or topical or orally administered small molecules (JAKi) with a broader mode of action by modulating gene expression of important pro-inflammatory cytokines in CNPG [67].
7.1 Agents Targeting Opioid Receptors
Clinical phase II and III trials investigating the efficacy and safety of nalbuphine, a κ-opioid receptor agonist and μ-opioid receptor antagonist, in CNPG are now completed (NCT02174432, NCT02174419, NCT03497975). Results of the phase II RCT (NCT02174419) have already been published [68]. Here, 62 patients were randomized 1:1:1 to receive nalbuphine extended-release (NAL-ER) tablets 81 mg, 162 mg or placebo twice daily for a treatment duration of 8 weeks. After 10 weeks of treatment, 44.4% (n = 8) (NAL-ER 162 mg twice daily; p = 0.32) and 27.3 % (n = 6; NAL-ER 81 mg twice daily; p = 0.78) of patients met the primary endpoint, which was a decrease of pruritus (as measured with 7-day PP-NRS) ≥ 30% from baseline (compared with 36.4%, n = 8 patients in the placebo group). In the open-label extension study, further improvements of pruritus and healing of skin lesions have been observed (measured with the Prurigo Activity Score). TEAEs comprised gastrointestinal disorders like nausea and general disorders like headache, fatigue and dizziness.
7.2 Agents Targeting the IL-31 Receptor A/Oncostatin M Receptor β
IL-31 is regarded as one of the most important Th2 cytokines in the pathophysiology of CNPG and chronic pruritus in general [69]. IL-31 binds to a heterodimeric receptor consisting of IL-31A and oncostatin M receptor β (OSMRβ) and thereby modulates neuroimmune signaling in CNPG and other pruritic diseases like atopic dermatitis [32, 70]. Nemolizumab is a monoclonal antibody that inhibits IL31RA. It is approved in Japan as AD add-on therapy in patients aged ≥ 13 years, when prior therapies have been insufficiently effective [70]. Nemolizumab is currently (June 2023) the only systemic therapy that is under investigation in CNPG in three phase III RCTs (NCT05052983, NCT04204616, NCT04501666; see ClinicalTrials.gov, EudraCT clinicaltrialsregister.eu). Results of the phase III RCT OLYMPIA 2 (NCT04501679) have been published as an abstract recently [71]. A total of 274 patients with moderate-to-severe CNPG participated in the global study. Of these, 183 received nemolizumab in a bodyweight-dependent dosage after a loading dose of 60 mg (< 90 kg bodyweight: 30 mg Q4W; ≥ 90 kg bodyweight: 60 mg Q4W) for 16 weeks, while 91 patients received placebo. A concomitant therapy with TCS or TCI was not allowed. After 16 weeks, both primary endpoints (i.e., proportion of patients with an improvement of pruritus ≥ 4 points as measured with the weekly average PP-NRS and proportion of patients with IGA success) were met (p < 0.0001). An itch reduction ≥ 4 points was achieved in 56.3% of nemolizumab-treated patients (weekly average PP-NRS) after a treatment duration of 16 weeks compared with 20.9% of patients that received placebo. IGA success was observed in 37.7% of patients in the nemolizumab group compared with 11.0% in the placebo group. The safety profile was similar to the phase II trial results [71].
The results of a 12-week, double-blind, phase II RCT (NCT03181503) also showed a fast improvement in pruritus intensity (PP-NRS − 19.5% vs − 5.8% [placebo]; p = 0.014) and sleep within 48 hours in nemolizumab-treated patients [72, 73]. The trial investigated 70 adult patients with moderate-to-severe CNPG and severe pruritus (NRS ≥ 7/10 points), of which 34 patients were treated with 0.5 mg nemolizumab per kilogram of bodyweight SC at baseline, week 4 and week 8; 36 patients received placebo [72]. After a treatment period of 4 weeks, PP-NRS was reduced by 4.5 points (nemolizumab) compared with 1.7 points (placebo group; p < 0.001) [72]. At this time, almost 30% of patients in the nemolizumab arm indicated (almost) no pruritus and 24% of patients achieved ≥ 75% healed skin lesions (vs 11% in the placebo arm) [72]. AEs were reported in about 70% of patients in both groups (severe AEs: n = 4 [nemolizumab]; n = 3 [placebo]). The most common AEs in the nemolizumab group were gastrointestinal symptoms (21% of patients), musculoskeletal or connective-tissue symptoms (18% of patients) and bronchitis (6% of patients) [72].
The monoclonal antibody vixarelimab (KPL-716) inhibits OSM and IL-31 signaling by binding to OSMRβ and has shown a fast decrease in pruritus intensity and a good improvement of skin lesions in two phase II RCTs (NCT03816891, NCT03858634). Sofen et al. report on 50 patients who were treated with vixarelimab (720 mg SC loading dose, then 360 mg SC weekly) or placebo for 8 weeks in Canada and the US (NCT03816891) [74]. In the vixarelimab group, a significant pruritus reduction (as measured by average weekly PP-NRS) compared with baseline at week 8 (−50.6%) was observed (placebo group: − 29.4%; 95% CI for the difference − 40.8 to − 1.6; p = 0.03) [74]. At that time, a pruritus reduction ≥ 4 points (PP-NRS) was achieved by 52.2% (vixarelimab) versus 30.8% (placebo) of patients (p = 0.11) [74]. Moreover, IGA stage was 0 or 1 in 30.4% of patients in the vixarelimab group (vs 7.7% in the placebo group; p < 0.03) at week 8 [74]. TEAEs in the vixarelimab arm comprised upper respiratory tract infections (21.7% of patients), nasopharyngitis (13.0% of patients), nummular eczema and urticaria (each 8.7% of patients) [74].
7.3 Topical and Systemic Agents Targeting the Janus Kinase Family
JAKi are small molecules that interfere with intracellular Janus kinase/signal transducer and activator of transcription (JAK/STAT) signaling pathways by inhibiting one or several members of the JAK family (intracellular tyrosine kinases JAK1, JAK2, JAK3, TYK2) with different relative selectivity. Many cytokines that mediate important immuno- and neuromodulating pathways in CNPG (e.g., IL-4, IL-13, thymic stromal lymphopoietin, IL-31, IL-17, IL-23) rely on JAK-STAT pathways [55, 59, 67, 75, 76].
They have proven effective in several other dermatological diseases (e.g., vitiligo, AD, psoriasis) [67]. For example, orally administered JAKi are approved in adult patients with moderate-to-severe AD (abrocitinib, baricitinib, upadacitinib) [77,78,79].
Ruxolitinib is the only approved topical JAKi to date (EMA approval: patients aged ≥ 12 years with non-segmental vitiligo with face involvement) [80]. In AD, 40.4%/42.7% (ruxolitinib 0.75% cream) and 52.2%/50.7% (ruxolitinib 1.5% cream), respectively, achieved an improvement of pruritus (PP-NRS) ≥ 4 points (placebo: 15.4%/16.3% of patients) at week 8 (TRuE-AD1/TRuE-AD2) [81] (all p < 0.001). Ruxolitinib cream is currently being tested in CNPG patients in two phase III RCTs (TRuE-PN1/NCT05755438; TRuE-PN2/NCT05764161); results have not yet been published.
Two systemic JAK1 inhibitors (INCB054707/povorcitinib [NCT05061693]; abrocitinib [NCT05038982]) are currently in phase II studies. In CNPG, efficacy and safety data of these two compounds are not yet available. There is one case report in the literature dealing with a CNPG patient who was successfully treated with abrocitinib and did not respond to dupilumab [82]. In AD, abrocitinib is known for rapid relief of pruritus within a few days. In JADE-MONO1, a double-blind, placebo-controlled phase III RCT, a significant pruritus reduction (PP-NRS ≥ 4 points) was observed in 20% (p = 0.0004)/46% (p < 0.0001) (abrocitinib 100 mg/200 mg; placebo: 3%) at week 2 and 32% (p = 0.0251)/59% (p < 0.0001) (placebo 17%) of patients at week 4 [83]. Here, reported TEAEs were nasopharyngitis, atopic dermatitis, nausea and headache [83].
Moreover, the immunoglobulin G1κ monoclonal antibody CDX-0159 (barzolvolimab) is being clinically tested in a double-blind, placebo-controlled, phase I RCT (NCT04944862). It may reduce pruritus in CNPG by inhibiting the KIT receptor, which may lead to mast cell depletion in the dermis as observed in mouse models [84].
8 Expert Opinion
Chronic prurigo is one representative example in dermatology where a misconception and terminology confusion has hampered the scientific elaboration for decades. In the past 15 years, this was corrected beginning with advocating for disease awareness [85], definition of a separate disease category in the IFSI classification [86] and first morphological [40] and clinical [58] studies. The first clinical trials evaluating novel substances were started 10 years ago (nalbuphine, NCT02174419). Since then, several milestones have been made in disease understanding and, corresponding to this, in improved medical care such as first transcriptomic analysis [26], first terminology [1], first guideline [47] and first therapy approval in 2022 (dupilumab). Many translational studies contributed to the understanding of the pathogenesis of this global disease, including ethnic variations. Many phase II/III studies have been initiated with different therapy targets such as Th2 interleukins (especially IL-4, IL-13, IL-31), the JAK/STAT pathway and neuroreceptors (NK1, opioid receptors). Positive study outcomes validated the pathophysiological role of the corresponding targets.
As patients suffer greatly and are frustrated about the lack of efficacy of available therapies and lack of approval of efficient therapies, there is still a high unmet need. There is a trend of patients avoiding contact with healthcare professionals or patient groups, and so much effort is needed to reach patients for dissemination of information on release of guidelines, studies or patient activities. Accordingly, a patient advocacy group led by physicians was founded 10 years ago. The Prurigo Nodularis League has since reached out to patients worldwide on social platforms.
The approval of new effective therapies for CNPG is most important for patients and health care professionals.
The approval of dupilumab for adult patients with moderate-to-severe CNPG in 2022 has been a major milestone since dupilumab represents the first approved systemic therapy for CNPG. Other therapies such as nemolizumab are expected to be approved soon. The approval of JAKi will probably take some time, but will provide faster itching relief in patients who need a particularly rapid response to therapy.
As we are in a highly innovative area with coverage of rare and neglected disease (such as hidradenitis suppurativa, vitiligo and others), drugs already approved for other indications are also reported to be effective in CNPG. Tofacitinib and tralokinumab are recent examples [87, 88]. The broad range of identified targets, current case observations and initiated trials offers the possibility of more drug approvals in the near future.
However, a lot of research questions remain, such as the natural course of the disease and the molecular and treatment response differences between people of different ethnicities. Further research including international collaborations with initiation of registries and translational studies are needed. Fortunately, this seems to be already underway.
References
Pereira MP, Steinke S, Zeidler C, Forner C, Riepe C, Augustin M, et al. European academy of dermatology and venereology European prurigo project: expert consensus on the definition, classification and terminology of chronic prurigo. J Eur Acad Dermatol Venereol. 2018;32:1059–65. https://doi.org/10.1111/jdv.14570.
Ständer S. How to define chronic pruritus: symptom or disease? Exp Dermatol. 2019;28:1461–5. https://doi.org/10.1111/exd.13953.
Schürmann CM, Schedel F, Plewig G, Löser C, Ständer H, Ständer S. Nihil certum: Historische Entwicklung des Begriffs Prurigo [Nihil certum: historical development of the term prurigo]. Hautarzt. 2014;65(8):674–83. https://doi.org/10.1007/s00105-014-2752-0.
Misery L. Chronic prurigo. Br J Dermatol. 2022;187:464–71. https://doi.org/10.1111/bjd.21698.
Gründel S, Pereira MP, Storck M, Osada N, Schneider G, Ständer S, Zeidler C. Analysis of 325 patients with chronic nodular prurigo: clinics, burden of disease and course of treatment. Acta Derm Venereol. 2020;100:adv00269. https://doi.org/10.2340/00015555-3571.
Pereira MP, Hoffmann V, Weisshaar E, Wallengren J, Halvorsen JA, Garcovich S, et al. Chronic nodular prurigo: clinical profile and burden. A European cross-sectional study. J Eur Acad Dermatol Venereol. 2020;34:2373–83. https://doi.org/10.1111/jdv.16309.
Augustin M, Garbe C, Hagenström K, Petersen J, Pereira MP, Ständer S. Prevalence, incidence and presence of comorbidities in patients with prurigo and pruritus in Germany: a population-based claims data analysis. J Eur Acad Dermatol Venereol. 2021;35:2270–6. https://doi.org/10.1111/jdv.17485.
Huang AH, Canner JK, Khanna R, Kang S, Kwatra SG. Real-world prevalence of prurigo nodularis and burden of associated diseases. J Invest Dermatol. 2020;140:480-483.e4. https://doi.org/10.1016/j.jid.2019.07.697.
Boozalis E, Tang O, Patel S, Semenov YR, Pereira MP, Stander S, et al. Ethnic differences and comorbidities of 909 prurigo nodularis patients. J Am Acad Dermatol. 2018;79:714-719.e3. https://doi.org/10.1016/j.jaad.2018.04.047.
Alkon N, Assen FP, Arnoldner T, Bauer WM, Medjimorec MA, Shaw LE, et al. Single-cell RNA sequencing defines disease-specific differences between chronic nodular prurigo and atopic dermatitis. J Allergy Clin Immunol. 2023. https://doi.org/10.1016/j.jaci.2023.04.019.
Deng J, Parthasarathy V, Marani M, Bordeaux Z, Lee K, Trinh C, et al. Extracellular matrix and dermal nerve growth factor dysregulation in prurigo nodularis compared to atopic dermatitis. Front Med (Lausanne). 2022;9:1022889. https://doi.org/10.3389/fmed.2022.1022889.
Storck M, Sandmann S, Bruland P, Pereira MP, Steinke S, Riepe C, et al. Pruritus Intensity Scales across Europe: a prospective validation study. J Eur Acad Dermatol Venereol. 2021;35:1176–85. https://doi.org/10.1111/jdv.17111.
Pereira MP, Zeidler C, Nau T, Bobko S, Evers AWM, Garcovich S, et al. Position Statement: Linear prurigo is a subtype of chronic prurigo. J Eur Acad Dermatol Venereol. 2019;33:263–6. https://doi.org/10.1111/jdv.15275.
Zeidler C, Pereira MP, Ständer S. Chronic prurigo: similar clinical profile and burden across clinical phenotypes. Front Med (Lausanne). 2021;8: 649332. https://doi.org/10.3389/fmed.2021.649332.
Brenaut E, Halvorsen JA, Dalgard FJ, Lien L, Balieva F, Sampogna F, et al. The self-assessed psychological comorbidities of prurigo in European patients: a multicentre study in 13 countries. J Eur Acad Dermatol Venereol. 2019;33:157–62. https://doi.org/10.1111/jdv.15145.
Zeidler C, Pereira MP, Dugas M, Augustin M, Storck M, Weyer-Elberich V, et al. The burden in chronic prurigo: patients with chronic prurigo suffer more than patients with chronic pruritus on non-lesional skin: a comparative, retrospective, explorative statistical analysis of 4,484 patients in a real-world cohort. J Eur Acad Dermatol Venereol. 2021;35:738–43. https://doi.org/10.1111/jdv.16929.
Pereira MP, Zeidler C, Wallengren J, Halvorsen JA, Weisshaar E, Garcovich S, et al. Chronic nodular prurigo: a European cross-sectional study of patient perspectives on therapeutic goals and satisfaction. Acta Derm Venereol. 2021;101:adv00403. https://doi.org/10.2340/00015555-3726.
Schneider G, Driesch G, Heuft G, Evers S, Luger TA, Ständer S. Psychosomatic cofactors and psychiatric comorbidity in patients with chronic itch. Clin Exp Dermatol. 2006;31:762–7. https://doi.org/10.1111/j.1365-2230.2006.02211.x.
Jørgensen KM, Egeberg A, Gislason GH, Skov L, Thyssen JP. Anxiety, depression and suicide in patients with prurigo nodularis. J Eur Acad Dermatol Venereol. 2017;31:e106–7. https://doi.org/10.1111/jdv.13827.
Schneider G, Zeidler C, Pereira MP, Ständer S. One third of chronic prurigo patients scratch automatically and in the absence of itch - a naturalistic study of 1142 patients. J Eur Acad Dermatol Venereol. 2022;36:e297–300. https://doi.org/10.1111/jdv.17777.
Oexle N, Rüsch N. Stigma—risikofaktor und Konsequenz suizidalen Verhaltens: implikationen für die Suizidprävention. [Stigma—risk factor and consequence of suicidal behavior: implications for suicide prevention]. Nervenarzt. 2018;89:779–83. https://doi.org/10.1007/s00115-017-0450-8.
Stumpf A, Ständer S, Warlich B, Fritz F, Bruland P, Pfleiderer B, et al. Relations between the characteristics and psychological comorbidities of chronic pruritus differ between men and women: women are more anxious than men. Br J Dermatol. 2015;172:1323–8. https://doi.org/10.1111/bjd.13492.
Shao Y, Zhu Y, Xiao Z, Shen Y, Dai B, Tang H, Wang D. RNA sequencing reveals the transcriptome profile of the atopic prurigo nodularis with severe itching. Exp Dermatol. 2023;32:30–40. https://doi.org/10.1111/exd.14678.
Calugareanu A, Specque F, Demouche S, Grolleau C, Dobos G, Merandet M, et al. Transcriptomic landscape of prurigo nodularis lesional skin CD3+ T cells using single-cell RNA-sequencing. J Invest Dermatol. 2023. https://doi.org/10.1016/j.jid.2023.05.011.
Sutaria N, Alphonse MP, Roh YS, Choi J, Parthasarathy V, Deng J, et al. Cutaneous transcriptomics identifies fibroproliferative and neurovascular gene dysregulation in prurigo nodularis compared with psoriasis and atopic dermatitis. J Invest Dermatol. 2022;142:2537–40. https://doi.org/10.1016/j.jid.2022.02.010.
Tsoi LC, Hacini-Rachinel F, Fogel P, Rousseau F, Xing X, Patrick MT, et al. Transcriptomic characterization of prurigo nodularis and the therapeutic response to nemolizumab. J Allergy Clin Immunol. 2022;149:1329–39. https://doi.org/10.1016/j.jaci.2021.10.004.
Agelopoulos K, Renkhold L, Wiegmann H, Dugas M, Süer A, Zeidler C, et al. Transcriptomic, epigenomic, and neuroanatomic signatures differ in chronic prurigo, atopic dermatitis, and brachioradial pruritus. J Invest Dermatol. 2023;143:264-272.e3. https://doi.org/10.1016/j.jid.2022.08.042.
Hashimoto T, Nattkemper LA, Kim HS, Kursewicz CD, Fowler E, Shah SM, et al. Dermal periostin: a new player in itch of prurigo nodularis. Acta Derm Venereol. 2021;101:adv00375. https://doi.org/10.2340/00015555-3702.
Patel JR, Joel MZ, Lee KK, Kambala A, Cornman H, Oladipo O, et al. Single-cell RNA sequencing reveals dysregulated fibroblast subclusters in prurigo nodularis. bioRxiv 2023. https://doi.org/10.1101/2023.01.29.526050.
Hashimoto T, Okuno S, Okuzawa M, Satoh T. Increased sensitivity to touch-evoked itch (punctate hyperknesis) in prurigo nodularis and type 2 inflammation: a cross-sectional pilot study. J Eur Acad Dermatol Venereol. 2023;37:e789–91. https://doi.org/10.1111/jdv.18942.
Liu T, Chu Y, Li S, Wang Y, Zhong X, Fang H, Qiao J. Myeloid dendritic cells are increased in the lesional skin and associated with pruritus in patients with prurigo nodularis. MedComm. 2020;2023(4): e204. https://doi.org/10.1002/mco2.204.
Hashimoto T, Nattkemper LA, Kim HS, Kursewicz CD, Fowler E, Shah SM, et al. Itch intensity in prurigo nodularis is closely related to dermal interleukin-31, oncostatin M, IL-31 receptor alpha and oncostatin M receptor beta. Exp Dermatol. 2021;30:804–10. https://doi.org/10.1111/exd.14279.
Parthasarathy V, Cravero K, Deng J, Sun Z, Engle SM, Auxier AN, et al. Circulating plasma IL-13 and periostin are dysregulated type 2 inflammatory biomarkers in prurigo nodularis: a cluster analysis. Front Med (Lausanne). 2022;9:1011142. https://doi.org/10.3389/fmed.2022.1011142.
Parthasarathy V, Cravero K, Xu L, Deng J, Sun Z, Engle SM, et al. The blood proteomic signature of prurigo nodularis reveals distinct inflammatory and neuropathic endotypes: a cluster analysis. J Am Acad Dermatol. 2023;88:1101–9. https://doi.org/10.1016/j.jaad.2023.01.042.
Ju T, Labib A, Nattkemper L, Engle S, Auxier A, Hahn N, et al. Serum interleukin-13 and caspase 8 are elevated in prurigo nodularis. Acta Derm Venereol. 2023;103:adv00861. https://doi.org/10.2340/actadv.v103.4804.
Chaowattanapanit S, Wongjirattikarn R, Chaisuriya N, Ungarreevittaya P, Poosekeaw P, Winaikosol K, et al. Increased IL-31 expression in serum and tissue protein in prurigo nodularis. Ther Adv Chronic Dis. 2022;13:20406223221112560. https://doi.org/10.1177/20406223221112561.
Sutaria N, Alphonse MP, Marani M, Parthasarathy V, Deng J, Wongvibulsin S, et al. Cluster analysis of circulating plasma biomarkers in prurigo nodularis reveals a distinct systemic inflammatory signature in African Americans. J Invest Dermatol. 2022;142:1300-1308.e3. https://doi.org/10.1016/j.jid.2021.10.011.
Pereira MP, Pogatzki-Zahn E, Snels C, Vu T-H, Üçeyler N, Loser K, et al. There is no functional small-fibre neuropathy in prurigo nodularis despite neuroanatomical alterations. Exp Dermatol. 2017;26:969–71. https://doi.org/10.1111/exd.13343.
Bobko S, Zeidler C, Osada N, Riepe C, Pfleiderer B, Pogatzki-Zahn E, et al. Intraepidermal nerve fibre density is decreased in lesional and inter-lesional prurigo nodularis and reconstitutes on healing of lesions. Acta Derm Venereol. 2016;96:404–6. https://doi.org/10.2340/00015555-2232.
Schuhknecht B, Marziniak M, Wissel A, Phan NQ, Pappai D, Dangelmaier J, et al. Reduced intraepidermal nerve fibre density in lesional and nonlesional prurigo nodularis skin as a potential sign of subclinical cutaneous neuropathy. Br J Dermatol. 2011;165:85–91. https://doi.org/10.1111/j.1365-2133.2011.10306.x.
Pogatzki-Zahn EM, Pereira MP, Cremer A, Zeidler C, Dreyer T, Riepe C, et al. Peripheral sensitization and loss of descending inhibition is a hallmark of chronic pruritus. J Invest Dermatol. 2020;140:203-211.e4. https://doi.org/10.1016/j.jid.2019.05.029.
Choragudi S, Yosipovitch G. Prurigo nodularis is highly linked with neural sensitization disorders of pain among hospitalized adults in the United States—National inpatient sample 2016–2019. Br J Dermatol. 2023. https://doi.org/10.1093/bjd/ljad121.
Pereira MP, Agelopoulos K, Köllner J, Neufang G, Schmelz M, Ständer S. Selective nerve fibre activation in patients with generalized chronic pruritus: hint for central sensitization? Acta Derm Venereol. 2019;99:1009–15. https://doi.org/10.2340/00015555-3261.
Vasavda C, Wan G, Szeto MD, Marani M, Sutaria N, Rajeh A, et al. A polygenic risk score for predicting racial and genetic susceptibility to prurigo nodularis. J Invest Dermatol. 2023. https://doi.org/10.1016/j.jid.2023.04.033.
Pölking J, Zeidler C, Schedel F, Osada N, Augustin M, Metze D, et al. Prurigo Activity Score (PAS): validity and reliability of a new instrument to monitor chronic prurigo. J Eur Acad Dermatol Venereol. 2018;32:1754–60. https://doi.org/10.1111/jdv.15040.
Zeidler C, Pereira MP, Augustin M, Spellman M, Ständer S. Investigator's global assessment of chronic prurigo: a new instrument for use in clinical trials. Acta Derm Venereol. 2021;101:adv00401. https://doi.org/10.2340/00015555-3701.
Ständer S, Pereira MP, Berger T, Zeidler C, Augustin M, Bobko S, et al. IFSI-guideline on chronic prurigo including prurigo nodularis. Itch. 2020;5:e42–e42. https://doi.org/10.1097/itx.0000000000000042.
Massa MC, Connolly SM. Bullous pemphigoid with features of prurigo nodularis. Arch Dermatol. 1982;118:937–9.
Hanumaiah B, Joseph JM. Role of dermoscopy in the diagnosis of hypertrophic lichen planus and prurigo nodularis. Indian J Dermatol. 2019;64:341–5. https://doi.org/10.4103/ijd.IJD_123_18.
Müller S, Ständer S, Naatz M, Augustin M, Steinke S. Cost-effectiveness and quality of specialized and routine care in a german cohort of patients with chronic pruritus. Acta Derm Venereol. 2023;103:adv4868. https://doi.org/10.2340/actadv.v103.4868.
Reich A, Chatzigeorkidis E, Zeidler C, Osada N, Furue M, Takamori K, et al. Tailoring the Cut-off values of the visual analogue scale and numeric rating scale in itch assessment. Acta Derm Venereol. 2017;97:759–60. https://doi.org/10.2340/00015555-2642.
Finlay AY, Khan GK. Dermatology life quality index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210–6. https://doi.org/10.1111/j.1365-2230.1994.tb01167.x.
Desai NS, Poindexter GB, Monthrope YM, Bendeck SE, Swerlick RA, Chen SC. A pilot quality-of-life instrument for pruritus. J Am Acad Dermatol. 2008;59:234–44. https://doi.org/10.1016/j.jaad.2008.04.006.
Snaith RP. The hospital anxiety and depression scale. Health Qual Life Outcomes. 2003;1:29. https://doi.org/10.1186/1477-7525-1-29.
Labib A, Ju T, Vander Does A, Yosipovitch G. Immunotargets and therapy for prurigo nodularis. Immunotargets Ther. 2022;11:11–21. https://doi.org/10.2147/ITT.S316602.
Yosipovitch G, Mollanazar N, Ständer S, Kwatra SG, Kim BS, Laws E, et al. Dupilumab in patients with prurigo nodularis: two randomized, double-blind, placebo-controlled phase 3 trials. Nat Med. 2023;29:1180–90. https://doi.org/10.1038/s41591-023-02320-9.
Wong SS, Goh CL. Double-blind, right/left comparison of calcipotriol ointment and betamethasone ointment in the treatment of prurigo nodularis. Arch Dermatol. 2000;136:807–8. https://doi.org/10.1001/archderm.136.6.807.
Siepmann D, Lotts T, Blome C, Braeutigam M, Phan NQ, Butterfass-Bahloul T, et al. Evaluation of the antipruritic effects of topical pimecrolimus in non-atopic prurigo nodularis: results of a randomized, hydrocortisone-controlled, double-blind phase II trial. Dermatology. 2013;227:353–60. https://doi.org/10.1159/000355671.
Williams KA, Huang AH, Belzberg M, Kwatra SG. Prurigo nodularis: pathogenesis and management. J Am Acad Dermatol. 2020;83:1567–75. https://doi.org/10.1016/j.jaad.2020.04.182.
Ständer HF, Elmariah S, Zeidler C, Spellman M, Ständer S. Diagnostic and treatment algorithm for chronic nodular prurigo. J Am Acad Dermatol. 2020;82:460–8. https://doi.org/10.1016/j.jaad.2019.07.022.
Dupilumab SM. First global approval. Drugs. 2017;77:1115–21. https://doi.org/10.1007/s40265-017-0768-3.
Fachler T, Maria Faitataziadou S, Molho-Pessach V. Dupilumab for pediatric prurigo nodularis: a case report. Pediatr Dermatol. 2021;38:334–5. https://doi.org/10.1111/pde.14464.
Fang H-Y, Lian C-H. The effectiveness and safety of dupilumab in the management of refractory prurigo nodularis in 45 Chinese patients: a real-life observational study. J Dermatol. 2023. https://doi.org/10.1111/1346-8138.16803.
Giovannini M, Mori F, Oranges T, Ricci S, Barni S, Canessa C, et al. Dupilumab treatment of prurigo nodularis in an adolescent. Eur J Dermatol. 2021;31:104–6. https://doi.org/10.1684/ejd.2020.3947.
Husein-ElAhmed H, Steinhoff M. Dupilumab in prurigo nodularis: a systematic review of current evidence and analysis of predictive factors to response. J Dermatolog Treat. 2022;33:1547–53. https://doi.org/10.1080/09546634.2020.1853024.
Damsky W, King BA. JAK inhibitors in dermatology: the promise of a new drug class. 2017.
Weisshaar E, Szepietowski JC, Bernhard JD, Hait H, Legat FJ, Nattkemper L, et al. Efficacy and safety of oral nalbuphine extended release in prurigo nodularis: results of a phase 2 randomized controlled trial with an open-label extension phase. J Eur Acad Dermatol Venereol. 2022;36:453–61. https://doi.org/10.1111/jdv.17816.
Datsi A, Steinhoff M, Ahmad F, Alam M, Buddenkotte J. Interleukin-31: The “itchy” cytokine in inflammation and therapy. Allergy. 2021;76:2982–97. https://doi.org/10.1111/all.14791.
Keam SJ. Nemolizumab: first approval. Drugs. 2022;82:1143–50. https://doi.org/10.1007/s40265-022-01741-z.
Ständer S, Yosipovitch G, Legat F. et al. 436 Nemolizumab monotherapy was associated with significant improvements in prurigo activity score in adult patients with moderate-to-severie prurigo nodularis: results form a phase 3 trial (OLYMPIA 2). Br J Dermatol. Suppl. 3. https://doi.org/10.1093/bjd/ljad162.056.
Ständer S, Yosipovitch G, Legat FJ, Lacour J-P, Paul C, Narbutt J, et al. Trial of nemolizumab in moderate-to-severe prurigo nodularis. N Engl J Med. 2020;382:706–16. https://doi.org/10.1056/NEJMoa1908316.
Ständer S, Yosipovitch G, Lacour J-P, Legat FJ, Paul C, Reich A, et al. Nemolizumab efficacy in prurigo nodularis: onset of action on itch and sleep disturbances. J Eur Acad Dermatol Venereol. 2022;36:1820–5. https://doi.org/10.1111/jdv.18377.
Sofen H, Bissonnette R, Yosipovitch G, Silverberg JI, Tyring S, Loo WJ, et al. Efficacy and safety of vixarelimab, a human monoclonal oncostatin M receptor β antibody, in moderate-to-severe prurigo nodularis: a randomised, double-blind, placebo-controlled, phase 2a study. EClinicalMedicine. 2023;57: 101826. https://doi.org/10.1016/j.eclinm.2023.101826.
Williams KA, Roh YS, Brown I, Sutaria N, Bakhshi P, Choi J, et al. Pathophysiology, diagnosis, and pharmacological treatment of prurigo nodularis. Expert Rev Clin Pharmacol. 2020. https://doi.org/10.1080/17512433.2021.1852080.
Nakashima C, Yanagihara S, Otsuka A. Innovation in the treatment of atopic dermatitis: emerging topical and oral Janus kinase inhibitors. 2022.
EMA approval cibinqo. https://www.ema.europa.eu/en/medicines/human/EPAR/cibinqo.
EMA approval olumiant. https://www.ema.europa.eu/en/medicines/human/EPAR/olumiant.
EMA approval rinvoq. https://www.ema.europa.eu/en/medicines/human/EPAR/rinvoq.
EMA approval opzelura. https://www.ema.europa.eu/en/medicines/human/EPAR/opzelura.
Papp K, Szepietowski JC, Kircik L, Toth D, Eichenfield LF, Leung DYM, et al. Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: results from 2 phase 3, randomized, double-blind studies. J Am Acad Dermatol. 2021;85:863–72. https://doi.org/10.1016/j.jaad.2021.04.085.
Vander Does A, Yosipovitch G. Failure of dupilimab with severe prurigo nodularis that responded well to abrocitinib. Dermatitis. 2023. https://doi.org/10.1089/derm.2022.0065.
Simpson EL, Sinclair R, Forman S, Wollenberg A, Aschoff R, Cork M, et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet. 2020;396:255–66. https://doi.org/10.1016/S0140-6736(20)30732-7.
Terhorst-Molawi D, Hawro T, Grekowitz E, Kiefer L, Merchant K, Alvarado D, et al. Anti-KIT antibody, barzolvolimab, reduces skin mast cells and disease activity in chronic inducible urticaria. Allergy. 2023;78:1269–79. https://doi.org/10.1111/all.15585.
Zeidler C, Ständer S. The pathogenesis of Prurigo nodularis—’Super-Itch’ in exploration. Eur J Pain. 2016;20:37–40. https://doi.org/10.1002/ejp.767.
Ständer S, Weisshaar E, Mettang T, Szepietowski JC, Carstens E, Ikoma A, et al. Clinical classification of itch: a position paper of the International Forum for the Study of Itch. Acta Derm Venereol. 2007;87:291–4. https://doi.org/10.2340/00015555-0305.
Pezzolo E, Gambardella A, Guanti M, Bianchelli T, Bertoldi A, Giacchetti A, et al. Tralokinumab shows clinical improvement in patients with prurigo nodularis-like phenotype atopic dermatitis: a multicenter, prospective, open-label case series study. J Am Acad Dermatol. 2023. https://doi.org/10.1016/j.jaad.2023.04.056.
Liu T, Chu Y, Wang Y, Zhong X, Yang C, Bai J, et al. Successful treatment of prurigo nodularis with tofacitinib: the experience from a single center. Int J Dermatol. 2023;62:e293–5. https://doi.org/10.1111/ijd.16568.
Rosmarin D, Passeron T, Pandya AG, Grimes P, Harris JE, Desai SR, et al. Two phase 3, randomized, controlled trials of ruxolitinib cream for vitiligo. N Engl J Med. 2022;387:1445–55. https://doi.org/10.1056/NEJMoa2118828.
Shi VY, Bhutani T, Fonacier L, Deleuran M, Shumack S, Valdez H, et al. Phase 3 efficacy and safety of abrocitinib in adults with moderate-to-severe atopic dermatitis after switching from dupilumab (JADE EXTEND). 2022.
Ständer S, Kwon P, Hirman J, Perlman AJ, Weisshaar E, Metz M, Luger TA. Serlopitant reduced pruritus in patients with prurigo nodularis in a phase 2, randomized, placebo-controlled trial. J Am Acad Dermatol. 2019;80:1395–402. https://doi.org/10.1016/j.jaad.2019.01.052.
Tsianakas A, Zeidler C, Riepe C, Borowski M, Forner C, Gerss J, et al. Aprepitant in anti-histamine-refractory chronic nodular prurigo: a multicentre, randomized, double-blind, placebo-controlled, cross-over, phase-II trial (APREPRU). Acta Derm Venereol. 2019;99:379–85. https://doi.org/10.2340/00015555-3120.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Open Access funding enabled and organized by Projekt DEAL. This research was funded by European Academy of Dermatology and Venerology with a grant to Claudia Zeidler (PPRC-2020-21). S. Ständer was supported by the German Research Foundation (DFG; STA 1159/4-2, STA 1159/6-2; FOR2690-PruSEARCH Translational Pruritus Research). The funding source did not participate in study design and conduct, nor in the data collection, management, analysis and interpretation, nor in preparation, review or approval of the manuscript nor in the decision to submit the article for publication.
Conflict of interest
Svenja Müller is or was advisor, speaker or investigator for Galderma, Incyte Inc., Eli Lilly, Sanofi/Regeneron and LEO Pharma outside the submitted work. Claudia Zeidler is or was advisor, speaker or investigator for Novartis, Janssen, Pfizer, UCB, Lilly, AbbVie, Boehringer Ingelheim, Sanofi, Regeneron, Leo, Galderma, Dermasence, Beiersdorf, Unna Akademie. She was a consultant for Sanofi and Allmiral. All outside the submitted work. Sonja Ständer is an investigator for Celldex, Clexio, Dermasence, Galderma, GSK, Incyte, Kiniksa, Novartis, Sanofi, Trevi. She is a consultant and/or member of advisory boards for AbbVie, Almirall, Beiersdorf, Benevolent, Bionorica, Cara, Celgene, Clexio, DS Biopharma, Eli Lilly, Escient, Galderma, Kiniksa, Klinge Pharma, Leo Pharma, Maruho, Menlo, P.G. Unna Academy, Pfizer, Sanofi, Symbio Research, Trevi, Vanda, Vifor, WebMD.
Ethics approval
Not applicable.
Patient consent to participate
Not applicable.
Patient consent to publish
Consent for the publication of the patient photograph (Fig. 2) was obtained by the authors.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Author contributions
S. Müller and C. Zeidler contributed equally to this work. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Müller, S., Zeidler, C. & Ständer, S. Chronic Prurigo Including Prurigo Nodularis: New Insights and Treatments. Am J Clin Dermatol 25, 15–33 (2024). https://doi.org/10.1007/s40257-023-00818-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40257-023-00818-z