Abstract
To better guide Cedrus libani provenances movement to northerly and higher-altitude sites for afforestation, the limits of hardiness to cold should be better known. In this study, we quantified the cold hardiness of seven C. libani provenances sampled from a provenance trial in Ankara, Türkiye, a site located outside the natural range of distribution of the species. Visual damage observation and chlorophyll fluorometry screening methods were used to assess variation in cold hardiness (LT50, the temperature estimated at which 50% of the needle tissue is damaged) among populations. Overall, C. libani can tolerate winter temperatures down to − 21.5 °C. Even though there were significant differences in cold hardiness among populations, the maximum difference was only 1.9 °C between the most and the least cold-resistant provenances. Cold tolerance was generally greater for provenances that experienced colder temperatures in March and lower levels of total precipitation in their native ranges. We also conclude that the fluorometry method provides a more efficient and stable comparison of cold hardiness than visual observation for C. libani. The results of this study may be useful for assisted migration and breeding programs, as well as for developing guidelines for genetic materials transfer, to increase productivity or adaptability of C. libani.
Similar content being viewed by others
Introduction
While global warming causes milder winter temperatures on average, it is also expected to increase temperature variance and the probability of extreme weather events (Vavrus et al. 2006; Francis and Skific 2015). The intensity and duration of cold extremes are expected to persist in Europe (Vavrus et al. 2006; Kodra et al. 2011). Consequently, frost damage for trees will likely not disappear in a globally warmer climate and may become even more problematic. Therefore, tree species adapted to the future climate of Central European must be tolerant to increased summer drought stress and tolerant to present and future low air and soil temperatures in winter (Bachofen et al. 2016).
Populations or species that originated in Mediterranean climates are expected to be better adapted to the future climate of Central Europe than temperate-originated ones (Bachofen et al. 2016). Furthermore, when growing under drought conditions, Pinus sylvestris seedlings from Spanish and Turkish populations have been reported to perform better than seedlings from Central or Northern European populations (Taeger et al. 2013; Matias and Jump 2014; Semerci et al. 2017). Such results support expectations of a northward shift of Mediterranean tree species to Western and Central Europe. Even though drought tolerance is an important factor affecting trees survival under future climatic conditions in Central Europe, Mediterranean species might be vulnerable to other factors when they are exposed to more northerly climates, where frost might constrain the northerly expansion of Mediterranean populations to Central Europe, where winter temperatures are considerably lower than in the Mediterranean region. There is a general expectation to transfer C. libani to Central Europe in the near future (Güney et al. 2015), which requires a better understanding of the species' cold hardiness.
The distribution range of Lebanon cedar, Cedrus libani A. (Rich.), was wider than today, but forests have changed since the Late Glacial era (Hajar et al. 2010). Currently, it occurs naturally only at around 2000 and 400 ha in Lebanon and Syria, respectively (Khuri et al. 2000; Haroutunian et al. 2017). Although the same disturbances have continued in Anatolia (Asia Minor), only small populations have survived in a well-preserved state in disjoint areas; particularly, the almost inaccessible topography of the Taurus Mountains has prevented C. libani from extinction (Boydak 2003; Boydak and Çalıkoğlu 2008). Some scattered populations can be found in other parts of Anatolia. This disjoint distribution together with site conditions (i.e., elevation, microclimate, absence or rareness of associating tree species) leads to morphological, physiological, phenotypical and molecular differences between distinct provenances of C. libani (Dirik 2000; Kayıhan et al. 2006; Boydak and Çalıkoğlu 2008; Fady et al. 2008).
Cedrus libani typically grows between 800 and 2100 m. a.s.l., smaller stands may occur in lower (500–600 m a.s.l.) or higher (up to 2400 m a.s.l.) elevations. Mean yearly precipitation varies between 600 and 1200 mm (Boydak 2003). Prolonged summer droughts are common in its habitat, and they can withstand frost at about −20 and −25 °C in arboretums in Massachusetts, USA, and Bayreuth, Germany (Goor and Barney 1976; Messinger et al. 2015). Extremely cold winter temperatures of −35 °C may occur on the top of the Taurus Mountains in its natural distribution in Türkiye (Boydak 2003).
Because of its plasticity toward climate extremes, high survival rate and unique wood properties, large afforestation/reforestation efforts with C. libani are being made outside its natural range in Türkiye and other countries such as Italy, Iran and Bulgaria. Moreover, it has also been planted in parks and gardens in many European and South American countries (Boydak and Çalıkoğlu 2008). Messinger et al. (2015) indicated that C. libani has a high adaption to current and future climate conditions in Central Europe, highlighting its promising potential for establishing stable and productive forest stands in Central Europe. Similarly, Frischbier et al. (2019) reported that C. libani has performed well in sites established on clear-cuts in Germany, Austria and Switzerland, which represent a broad range of geological and climatic conditions typical for Central Europe.
Cedrus libani has been widely used in afforestation/reforestation activities in Türkiye and Lebanon, as a major species for ecosystem restoration and increasing forest cover during the last three decades. As a result, many cedar stands were established, mainly through planting, in the Aegean, Marmara, Black Sea and Inner and Eastern Anatolian regions of Türkiye, doubling the planted area to about 47,000 ha. Additionally, there are 11,000 ha of pure natural stands of the species in Türkiye (Boydak 2020). Simulations of C. libani future geographic distribution for the year 2100 on the entire Eastern Mediterranean region show that only a few areas in Syrian and Lebanon may allow its survival of the species in its native range (Hajar et al. 2010). Under future climate scenarios (i.e., RCP 4.5 and RCP 8.5), the area suitable for C. libani would increase outside its natural range, especially in Central–Northern Turkish, while, on the other hand, Southern Turkish ranges will lose potential distribution at lower elevations, pushing the species to higher-elevation sites (López-Tirado et al. 2021). Climate change would be a driver for assisted migration for the species, extending the distribution to a larger area than the present distribution.
Cold hardiness is an important trait reflecting adaptation to climate. It can be assessed by evaluating the freezing damage after natural frost events in field trials, but this method possesses limitations related to uncontrolled conditions. A better solution is to subject samples to different freezing temperatures under controlled conditions and to evaluate the freezing damage in those samples (Burr et al. 2001). Cold stress results in various observable or measurable symptoms of injury including the death of whole plants, visible necrosis of specific tissues and organs; or less obvious cellular symptoms that can be detected by vital staining, osmotic responsiveness, chlorophyll fluorescence, or by measuring relative electrolyte leakage in the affected tissues. Visual observation (VO) and chlorophyll fluorometry (CF) are commonly used methods for screening cold hardiness (Burr et al. 2001; Prada et al. 2014).
Due to climate change, assisted migration of select populations of Cedrus libani may be required to preserve the species. However, it is unknown whether these populations possess the necessary cold hardiness to survive at higher latitudes and/or higher elevations where the minimum temperatures are colder than the populations currently experience. Therefore, the objectives of this study were to: (1) determine whether there is frost hardiness difference among provenances, (2) compare two screening methods (VO and CF) commonly used to assess frost hardiness and (3) determine relations between cold hardiness of provenances and the climate of their native ranges.
Materials and methods
Provenances
In Türkiye, wide-scale provenance tests of C. libani were established in 1989–1990. They were established with 35 different provenances at 16 different areas in the country. This study was based on the C. libani provenance trial that was established in 1990 in Ankara. The study site is out of the natural distribution range of C. libani and is characterized by cooler temperatures than other provenance trials. The provenance test includes 35 provenances, which represent the entire range of the species. The provenance trial was established with 2-year-old potted seedlings with 3.0 × 1.5 m spacing (Boydak and Çalıkoğlu 2008), using a randomized complete block design consisting of 35 provenances, 3 blocks and 16 trees per provenance per blocks (Gökdemir et al. 2012).
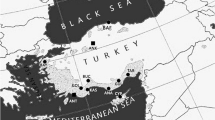
For this study, we selected seven provenances for cold hardiness assessment: Arslanköy (ARS), Çığlıkara (CIG), Ermenek (ERM), Göksun (GOK), Gölhisar (GOL), Niksar (NKS) and Sultandağı (SUL) (Fig. 1). The seven provenances were selected using survival data from the provenance trial carried out at age 20 years. Across all 35 provenances at age 20 years, mean survival was 88%, while survival of seven sampled provenances spanned between 56 and 94% (Table 1).
Geographic distribution of natural and planted stands of Cedrus libani (Adapted from (López-Tirado et al. 2021), including the location of provenance trial (black square, ANK) and provenances (black dots) locations
Meteorological data from provenances origin and study sites were obtained from the Turkish Meteorology Service using data from the nearest weather station (about 10 km) available (Türkiye Meteorological Service 2020). According to Emberger Bioclimatic Classification (Baylan and Ustaoğlu 2020), Mediterranean bioclimatic conditions characterized by summer drought index (S) are dominant at the provenances and study sites (i.e., S < 5). Nevertheless, the rainfall–temperature coefficient (Q) of ARS provenance (rainy) was higher than the rest (semiarid). Subzones classification indicates that winters of the provenances sites span between cold and extremely cold, while winters of the test site (ANK) are classified at an intermediate position, as very cold (Table 1).
Long-term minimum monthly temperature (percentile 5 of minimum monthly temperatures for 27–55 years of observations) of provenance origin and study sites is shown in Fig. 2. During winter, provenance origin sites ARS and ERM reached a minimum temperature above −15 °C, while provenance origin sites CIG, GOL and NKS, as well as the study site ANK, showed minimum temperatures ranging between −15 and −25 °C. Provenance origin sites SUL and GOK had the lowest temperatures, reaching below −25 °C during January and February.
Branch sampling and cold hardiness tests
The whole-plant freeze testing method was used to estimate frost damage (Burr et al. 2001). Distal parts of the branches of approximately 20 cm length were frozen at different temperatures in a programmable freezer. At the study site, branchlets (~ 20 cm in length) were collected from the southern middle part of the crown (fully exposed to the sun) from 10 trees per provenance (70 samples total). Dominant trees distributed over all three blocks were chosen. Sample collection was carried out using a ladder and a telescopic tree pruner in the middle of February 2019. Immediately after branchlets excision, samples were placed in plastic bags and sprayed with distilled water while kept in the dark in a cooler and transported to the laboratory where they were kept moist at a refrigerator at + 4 °C for one day until measurements started.
The day after sample excision, the whole branchlets were inserted in a low-temperature freezer equipped with a programmable controller (VötschVT3 4034). The control group was kept at + 4 °C. The freezing rate was kept to 5 °C/h until each target temperature (−15, −20, −22.5, −25 and −30 °C) was reached (Burr et al. 2001). Each target temperature was achieved independently to keep each treatment group at the target temperature for 5 h. After that, thawing was done by increasing temperature by 5 °C per hour until they reached room temperature.
Visual observation
After thawing, the remaining twigs of each freezing treatment were left for two weeks at a greenhouse temperature (+ 20 °C) to allow visible signs of freezing damage to develop. Freeze-injured tissue typically develops a brown or yellowish color from the oxidation of polyphenols (Lindén 2002). To improve the visibility of symptoms, water was sprayed on samples every three days. The cold injury was assessed visually by recording the percentage of discolored needles on branches two weeks after freezing tests (Burr et al. 2001; Prada et al. 2014). Visual observation (VO) scoring in needles was done by using a scale of 0 to 4 depending on the percentage of the foliar area damaged (0 = 0–20%; 1 = 21–40%; 2 = 41–60%; 3 = 61–80%; 4 = 81–100% of the foliar area damaged). For statistical analyses, the class value was used on each category (10, 30, 50, 70 and 90%, respectively).
Chlorophyll fluorometry
Chlorophyll fluorescence (CF) measurements provide information about the overall photosynthetic potential of the plant and its responses to stress or disturbances (Mohammed et al. 1995). The ratio of variable chlorophyll fluorescence to maximum chlorophyll fluorescence (Fv/Fm) is linearly correlated with the quantum yield of net photosynthesis. The parameter Fv/Fm was used for CF as it is highly correlated with low-temperature tolerance (Strand and Öquist 1985; Rose and Haase 2002). High values of Fv/Fm reveal undamaged tissue, while low values are indicative of freezing damage. Measurements of Fv/Fm were performed using a handheld chlorophyll fluorometer (Opti-Sciences OS30p). After thawing the twigs at each freezing treatment, needles were acclimated to the dark for at least 30 min before they were measured. From here and after, we will refer to Fv/Fm as CF.
Statistical analysis
A nonlinear model reflecting the relationship between temperature (expressed as positive values) and tissue damage (VO or CF) was fitted for each sample. After testing several sigmoidal curves, the model proposed by Kreyling et al. (2012) was selected:
where \({Y}_{T}\) is the tissue damage (VO or CF) at temperature T, \({Y}_{{\text{min}}}\) is the lower horizontal asymptotic value of the response variable, \({Y}_{{\text{max}}}\) is the upper horizontal asymptotic value of the response variable, k represents the steepness of the response curve and \({T}_{m}\) is the inflection point of the curve, which corresponds to LT50, the temperature at which 50% pf damage was observed (Pinheiro and Bates 2000).
The Statistical Analysis Software version 9.4 (SAS Institute Inc. Cary, NC) was used for all statistical analyses. To determine the relationships between LT50 and environmental variables (elevation, latitude and minimum temperatures of each winter month, January to March, including the average across all months), simple correlation analysis was performed for each environmental variable and LT50. Additionally, multiple linear regression was performed for modeling LT50 as a function of environmental variables. The model with significant parameter estimates with the lowest Akaike information criterion (AIC) was finally selected. In order to detect multicollinearity among explanatory variables, the variance inflation factor (VIF) was monitored, discarding variables included in the model with a VIF larger than 5 (Neter et al. 1996). Model parameters were selected using a stepwise selection procedure. Residuals were examined to ensure that statistical assumptions of normality and homoscedasticity were met. Nonlinear model fitting (PROC NLIN) was used to determine LT50 (relationship between temperature and tissue damage) and to model the relationship between LT50 and environmental variables. Analysis of variance, including Tukey multiple comparisons tests (PROC MIXED), was used to test the effects of provenances on LT50. All figures were produced using SigmaPlot version 14 (Systat Software, Inc. San Jose, CA).
Results
Cold hardiness variations among provenances
Overall, the two methods used to assess frost damage on C. libani showed similar results, indicating that lethal damage in needles started below −20 °C. There was a sharp increase in damage between −20 and −25 °C (Fig. 3).
There were significant differences in LT50 among C. libani provenances (P < 0.0001), and the values of LT50 differed among screening methods (P = 0.0072). Across provenances, LT50 of C. libani ranged between −23.4 and −21.5 °C, when evaluated with the VO method, and between −23.8 and −22.4 °C, when evaluated with the CF method. Independent of the screening method, the most and least frost-resistant provenances were NKS and ARS, respectively (Table 2).
Cold hardiness was related to the climate within the native ranges of the provenances. There was no correlation between LT50 and latitude of provenance origin, independent of the screening method (P = 0.218 and P = 0.198, for VO and CF, respectively). On the other hand, there was a significant nonlinear relationship between LT50 and elevation of provenance origin (P < 0.05), where LT50 declined sharply as elevation of origin site larger than 1650 m.a.s.l. Provenances originated on sites with elevations lower than 1550 m.a.s.l. displayed relatively constant and higher cold hardiness (Fig. 4).
There was a correlation between LT50 and the minimum temperature of January at the provenance origin (P < 0.05; R2 = 0.19 and R2 = 0.54, for VO and CF, respectively), and that relationship was nonlinear, decreasing frost hardiness as the minimum temperature of January was higher than −20 °C. Correlation between LT50 and the minimum temperature of all winter months (December to March, as well as the average of all winter months) was also tested, and even though minimum temperature of March showed the largest correlation (R2 = 0.92), we decided to use the model with minimum temperature of January (R2 = 0.91) as it has a stronger biological interpretation. Furthermore, LT50 was also correlated with total annual rainfall at the provenance origin (P < 0.0001), where cold hardiness was generally greater for provenances that experienced lower levels of precipitation in their native ranges (Fig. 5).
Relationship between LT50 determined using chlorophyll fluorescence (CF, upper panel) and visual observation of percent damage after 2 weeks (VO, bottom panel) methods and minimum temperature of January (left) and annual rainfall (right) of Cedrus libani provenances. Error bars represent standard error (n = 10)
Using data from CF determination method, a multiple nonlinear regression was fitted to describe the general effect of both total annual rainfall (62.7% of the explained variance) and minimum temperature of January (24.8% of the explained variance) of the origin site on LT50 (P < 0.0001; R2 = 0.88). Parameter estimates and fit statistics of the models that depict the relationships between LT50 (determined using CF), and environmental characteristics of provenance origin sites (elevation, total rainfall and minimum temperature of January), as well as an equation to estimate LT50 from the combined effect of those environmental variables, are shown in Table 3.
When all environmental variables were included into the model, elevation was not selected for the final linear model. Overall, cold hardiness increased (decreasing LT50) for provenances origin sites with a lower minimum temperature of January and with a lower total annual rainfall. The combined effect of both climatic variables is shown in Fig. 6.
Comparison of screening methods
Overall, mean LT50 assessed by VO and CF was −22.6 and −23.3 °C, a difference of about 0.7 °C between screening methods, implying that, in practice, both methods gave similar results for C. libani. Furthermore, there was a strong correlation between the two methods used to determine frost tolerance across provenances (P < 0.001; R2 = 0.79; Fig. 7).
Discussion
Cold hardiness variations among provenances
Species from cold climates are likely to have high resistance to frost than those from warmer climates. Consequently, a classification of climate that takes minimum temperatures into account is most likely to be correlated with the cold hardiness of species. The US Department of Agriculture (USDA) refined the method and adopted 11 cold hardiness zones. Plants have been ascribed hardiness ratings that are numerically equivalent to the lowest climatic zone in which they have been observed to survive. According to hardiness zones developed by USDA, C. libani is classified in Zone 7, having a minimum temperature of −17.7 °C as its zonal limit. However, practically all species have measured cold hardiness that exceed the limits defined by their particular cold hardiness zones. The discrepancy is about 6.9 °C for Zone 7. Therefore, we can extend the minimum temperature of Zone 7 to −24.6 °C (Bannister and Neuner 2001).
In our experiment, the frost hardiness of C. libani ranged from −23.8 (with CF) to −21.5 °C (with VO). Hence, our determinations of cold hardiness of C. libani are consistent with the cold hardiness zones made by the USDA. Our findings are also consistent with a previous study of Kreyling et al. (2015) who observed visual signs of natural frost damage on C. libani at the Ecological Botanical Garden of the University of Bayreuth, Germany, when temperature decreases to −23.7 °C in midwinter. The authors also measured cold tolerance (LT50 obtained by relative electrolyte leakage method) of C. libani between −18.7 and −23.3 °C in March. Based on those results and in our findings, we recommend that practitioners avoid planting C. libani in regions where the lowest temperatures drop below −21.5 °C (our observations indicated an LT50 of −21.5 °C in VO analysis). However, it is important to consider the absolute minimum temperature in GOL (Gölhisar) provenance, where C. libani naturally spreads and reaches as low as −33.5 °C (Table 1). Considering the natural habitat of C. libani, where temperatures can be exceptionally low, the duration of the exposure to these freezing temperatures might be shorter than the 5 h duration examined in our study. Consequently, the damage experienced by C. libani is likely influenced not only by the intensity of frost but also by the duration of exposure. Practitioners should take both factors into account when deciding on suitable planting locations for this species.
In this study, we found that cold hardiness patterns could largely be explained by the climate of the provenance origins which indicates that cold hardiness was genetically differentiated among populations. Subsequently, there is a potential for the selection of more frost-tolerant C. libani populations, which is an important advantage for a species that have to survive in harsh environments, especially in continental areas or at high altitudes. Nevertheless, the magnitude of provenance variation in midwinter cold tolerance was relatively small (1.9 °C between the most tolerant and the least tolerant provenances), when compared with other conifer species. For example, the range in average midwinter cold hardiness among 12 red spruce provenances was 8 to 10 °C and 14 to 18 °C among balsam fir provenances (DeHayes et al. 2001). Similarly, cold hardiness in winter differed by about 10 °C between provenances of black pine (Kreyling et al. 2012). The reason for the small variation in cold hardiness observed among provenance of C. libani may be low variation in geographic and climatic conditions across the range of distribution of the species.
Our results indicate that, independent of the screening method employed, NKS (Niksar-Çatalan) was the hardest, and ARS (Mersin-Arslanköy) was the least hardy provenance to cold. However, according to the randomly amplified polymorphic DNA (RAPD) analysis of 14 Turkish populations by Kayıhan et al. (2006), the parent population of NKS could be the Mediterranean population of ARS. In that study, the authors did not find a clear pattern for geography, as the two distantly located seed stands NKS and ARS (see Fig. 1) were genetically similar, suggesting that the northern population NKS may have been introduced from the southern ARS population in the past. According to historical records, the NKS population might be introduced to its current area of distribution around the second century B.C., being isolated from core populations in Türkiye (Fady et al. 2008). Nevertheless, the authors did not find genetic similarities between the NKS and ARS provenances. Our results indicated that ARS and NKS might be different provenances according to their different cold hardiness (Table 2).
Cold hardiness is generally greater at higher latitudes and altitudes, reflecting the decreasing temperature as latitude and altitude increase. This relationship has often been interpreted but is less frequently quantified (Bannister and Neuner 2001; Bannister 2007). Our results, however, showed no relationship between the cold hardiness and latitude of provenances origin of C. libani. Moreover, contradicting the general idea, provenances originated from lower altitudes generally displayed higher cold hardiness. Consistent with our result, other several studies also did not find a relationship between cold hardiness and latitude of provenances origin in Pinus albicaulis (Bower and Aitken 2006), Pinus pinaster (Prada et al. 2016) and Pinus brutia (Semerci et al. 2021).
Our results indicate that cold hardiness increased (decreased LT50) with decreasing annual rainfall (62.7% of the explained variance) and with decreasing minimum temperature of January (24.8% of the explained variance), suggesting that the selection of frost-tolerant provenances could be based on the current climatic conditions within the species’ ranges. Furthermore, the fact that cold hardiness patterns could be explained by the climate of the provenance origins indicates that cold hardiness was genetically differentiated among populations. A negative correlation between annual rainfall and cold hardiness in C. libani may indicate evidence that cold hardiness is physiologically related to drought tolerance in conifers. Drought and frost, for instance, lead to similar physiological responses in a coniferous forest tree aiming to prevent cellular dehydration. Thus, decreasing latitudes associated with increasing temperatures and decreasing rainfall may be increased both drought and cold hardiness in C. libani. This result is consistent with the study of Kreyling et al. (2015) who quantified cold tolerance (LT50 obtained by relative electrolyte leakage method) of 27 native and exotic (Northern Hemisphere) tree species. The authors concluded that, in general, cold tolerance increased with decreasing precipitation over the species’ ranges. Therefore, as suggested by Kreyling et al. (2015), we may imply that drought-tolerant Mediterranean species (or populations) might be preadapted to frost.
Cold tolerance of tree species or provenances is related to the extreme minimum temperatures occurring within the species’ ranges (Bannister 2007). Consistent with this, cold tolerance was generally greater for provenances that experienced colder January temperatures at the provenances’ origin in our study. Similarly, Kreyling et al. (2012) found significant correlations (R2 = 0.77 in the first winter and R2 = 0.80 in the second winter) between cold hardiness and mean minimum winter temperature of Pinus nigra provenances origins.
Comparison of screening methods
In the literature, the visual observation method generally is strongly correlated with the Chlorophyll fluorometry method, although, in general, visual scoring was less precise than the fluorometry method (Binder and Fielder 1996; L’Hirondelle et al. 2006; Peguero-Pina et al. 2008; Prada et al. 2014; Semerci et al. 2021). In our study, the two cold hardiness screening methods (VO and CF) compared in this study were well correlated and both methods led to similar LT50 values for each provenance. Visual scores estimated only a bit lower (0.7 °C) the level of damage compared to Chlorophyll fluorometry, implying that both methods provide similar accuracy, being comparable and useful tools for cold damage assessment. On the other hand, we measured frost damage 4 h after the freeze–thaw treatment using fluorometry and two weeks after the freeze–thaw treatment using visual scoring. Thus, the major advantages of Chlorophyll fluorescence over visual scoring are the shorter time to completion and the elimination of observer bias in visual scoring.
Conclusions
According to the results using the CF screening method, the most sensitive population can withstand up to −22.4 °C and the most tolerant population can tolerate a temperature of −23.8 °C. Our results imply that the cold hardiness of the needles is adaptive to long-term climatic conditions at the seed origin (genetic heritage). According to our results, CF is the most recommendable method for assessing cold hardiness since it was faster and more objective than the visual observation. From a practical standpoint, we recommend that C. libani should not be transferred to areas where winter temperature decreases to −21.5 °C and below, suggesting that a northward migration of Mediterranean C. libani to Central and Western Europe is not limited by frost damage as the minimum temperature of that region rarely decreases to −20 °C. However, under global warming conditions, drought may be a limiting factor in this region. Analyzing frost tolerance at a single point in time may not be enough to guide assisted migration efforts. Hence, our results should be supported by new studies which contain spring and autumn frost tolerance assessments.
References
Bachofen C, Wohlgemuth T, Ghazoul J, Moser B (2016) Cold temperature extremes during spring do not limit the range shift of Mediterranean pines into regions with intermittent frost. Funct Ecol 30:856–865. https://doi.org/10.1111/1365-2435.12581
Bannister P (2007) A touch of frost? Cold hardiness of plants in the Southern Hemisphere. NZ J Bot 45(1):1–33. https://doi.org/10.1080/00288250709509700
Bannister P, Neuner G (2001) Frost resistance and the distribution of conifers. New For 30:167–184
Baylan KA, Ustaoğlu, B (2020) Distribution of Types and Subtypes of The Mediterranean Bioclimatic Zones According to Emberger Bioclimatic Classification in Turkey. Ulusal Çevre Bilimleri Araştırma Dergisi, Sayı 3: 158–174. (in Turkish with an abstract in English)
Binder WD, Fielder P (1996) Chlorophyll fluorescence as an indicator of frost hardiness in white spruce seedlings from different latitudes. New For 11:233–253
Bower AD, Aitken SN (2006) Geographic and seasonal variation in cold hardiness of whitebark pine. Can J For Res 36:1842–1850
Boydak M (2003) Regeneration of Lebanon cedar (Cedrus libani A. Rich.) on karstic lands in Turkey. For Ecol Manag 178:231–243
Boydak M (2020) Sedirin (Cedrus libani A. Rich.) Tahribi Sonucu Toroslarda Oluşan Çıplak Karstik Alanların Ekim Yöntemiyle Yeniden Sedir Ormanlarına Dönüştürülmesinin Öyküsü, Orman ve Av Dergisi 5:20–30
Boydak M, Çalıkoğlu M (2008) Biology and Silviculture of Lebanon Cedar (Cedrus libani A. Rich.) Ogem-Vak Press, Ankara
Burr KE, Hawkings CDB, L’Hirondelle SJ, Binder W D, George MF, Repo T (2001) Methods for measuring cold hardiness of conifers. In: Bigras FJ, Colombo SJ (eds) Conifer cold hardiness. Kluwer Academic, Dordrecht, pp 369–401
DeHayes DH, Schaberg PG, Strimbeck GR (2001) Red Spruce (Picea rubens Sarg.) cold hardiness and freezing injury susceptibility. In: Bigras FJ, Colombo SJ (eds) Conifer cold hardiness, Kluwer Academic Publishers, Dordrecht, USA, pp 495–529
Dirik H (2000) Effet du stress hydrique osmotique sur la germination des graines chez les provenances de Ce`drus du liban (Cedrus libani A. Rich) d’origine Turque. Ann For Sci 57:361–367
Emberger L (1955) Une classification biogéographique des climats – Recherches et Travaux du Laboratoire de Botanique de la Faculté des Sciences de Montpellier, série Botanique 7:3–43
Fady B, Lefevre F, Vendramin GG, Ambert A, Regnier C, Bariteau M (2008) Genetic consequences of past climate and human impact on eastern Mediterranean Cedrus libani forests, implications for their conservation. Conserv Genet 9:85–95
Francis J, Skific N (2015) Evidence linking rapid Arctic warming to mid-latitude weather patterns. Phil Trans R Soc A 373:20140170. https://doi.org/10.1098/rsta.2014.0170
Frischbier N, Nikolova PS, Brang P, Klumpp R, Aas G,·Binder F (2019) Climate change adaptation with non‑native tree species in Central European forests: early tree survival in a multi-site field trial. European J Forest Research 138:1015–1032. https://doi.org/10.1007/s10342-019-01222-1
Goor AY, Barney CW (1976) Forest Tree Planting in Arid Zones. The Ronald Press Company, New York.
Gökdemir Ş, Örtel E, Çetinay Ş, Altun ZG, Karatay H, Boza A (2012) Results of Provenance trials of Taurus Cedar (Cedrus libani A. Rich) at the 20 years in Turkey. Central Anatolia Forestry Research Institute. Technical Bulletin No: 298 Ankara, Turkey
Güney A, Kerr D, Sökücü A, Zimmermann R, Küppers M (2015) Cambial activity and xylogenesis in stems of Cedrus libani A. Rich at different altitudes. Bot Stud 56(1):1–10
Hajar L, Francois L, Khater C, Jomaa I, Deque M, Cheddadi R (2010) Cedrus libani (A. Rich) distribution in Lebanon: past, present and future. CR Biol 333:622–630. https://doi.org/10.1016/j.crvi.2010.05.003
Haroutunian G, Chojnacky D C, El Riachy R, Chojnacky C C (2017) Reducing reforestation costs in Lebanon: adaptive field trials. Forests 8:169. https://doi.org/10.3390/f8050169
Kayıhan GC, Kaya Z, Kandemir G, Önde S (2006) The genetic structure of Cedrus libani (A. Rich) seed stands determined by random amplified polymorphic DNA markers. For Genet 12(3):181–190
Khuri S, Shmoury MR, Baalbaki R, Maunder M, Talhouk SN (2000) Conservation of the Cedrus libani populations in Lebanon: history, current status and experimental application of somatic embryogenesis. Biodivers Conserv 9(9):1261–1273
Kodra E, Steinhaeuser K, Ganguly AR (2011) Persisting cold extremes under 21st-century warming scenarios. Geophysical Research Letters, 38:L08705. https://doi.org/10.1029/2011GL047103
Kreyling J, Wiesenberg GLB, Thiel D, Wohlfart C, Huber G, Walter J, Jentsch A, Konnert M, Beierkuhnlein C (2012) Cold hardiness of Pinus nigra Arnold as influenced by geographic origin, warming, and extreme summer drought. Environ Exp Bot 78:99–108. https://doi.org/10.1016/j.envexpbot.2011.12.026
Kreyling J, Schmid S, Aas G (2015) Cold tolerance of tree species is related to the climate of their native ranges. J Biogeogr 42:156–166
Lindén L (2002) Measuring cold hardiness in woody plants. Dissertation, University of Helsinki
L’Hirondelle SJ, Simpson DG, Binder WD (2006) Overwinter storability of conifer planting stock: operational testing of fall frost hardiness. New For 32:307–321
López-Tirado J, Vessella F, Stephan J, Ayan S, Schirone B, Hidalgo PJ (2021) Effect of climate change on potential distribution of Cedrus libani A. Rich in the twenty-first century: an ecological niche modeling assessment. New Forests 52:363-376 https://doi.org/10.1007/s11056-020-09798-y
Matias L, Jump AS (2014) Impacts of predicted climate change on recruitment at the geographical limits of Scots pine. J Exp Bot 65:299–310
Messinger J, Güney A, Zimmermann R, Ganser B, Bachmann M, Remmele S, Aas G (2015) Cedrus libani: a promising tree species for Central European forestry facing climate change? Eur J For Res 134:1005–1017. https://doi.org/10.1007/s10342-015-0905-z
Mohammed GH, Binder WD, Gillies SL (1995) Chlorophyll fluorescence: A review of its practical forestry applications and instrumentation. Scan J For Res 10:383–410
Neter J, Kutner MH, Nachtsheim CJ, Wasserman W (1996) Applied linear statistical models (4th editio) Irwin
Prada E, Alía R, Climent J, Raquel D (2014) Seasonal cold hardiness in maritime pine assessed by different methods. Tree Genet Genomes 10:689–701 https://doi.org/10.1007/s11295-014-0714-4
Prada E, Climent J, Alía R, Díaz R (2016) Life-history correlations with seasonal cold hardiness in maritime pine. Am J Bot 103 (12):1–10
Peguero-Pina JJ, Morales F, Gil-Pelegrin E (2008) Frost damage in Pinus sylvestris L. stems assessed by chlorophyll fluorescence in cortical bark chlorenchyma. Ann For Sci 65:813
Pinheiro JC, Bates DM (2000) Mixed-effects models in S and S-plus. Springer Series in Statistics and Computing. Springer-Verlag, New York
Rose R, Haase D (2002) Chlorophyll fluorescence and variations in tissue cold hardiness in response to freezing stress in Douglasfir seedlings. New For 23:81–96
Semerci A, İmal B, Gonzalez-Benecke CA (2021) Intraspecific variability in cold tolerance in Pinus brutia sampled from two contrasting provenance trials. New For 52:621–637. https://doi.org/10.1007/s11056-020-09815-0
Semerci A, Semerci H, Çalişkan B, Çiçek N, Ekmekçi Y, Mencuccini M (2017) Morphological and physiological responses to drought stress of European provenances of Scots pine. Eur J Forest Res 136:91–104. https://doi.org/10.1007/s10342-016-1011-6
Strand M, Öquist G (1985) Inhibition of photosynthesis by freezing temperatures and high light levels in cold-acclimated seedlings of Scots pine (Pinus sylvestris). I. Effects on the light-limited and light-saturated rates of CO assimilation. Physiol Plant 64:425–430
Taeger S, Fussi B, Konnert M, Menzel A (2013) Large-scale genetic structure and drought-induced effects on European Scots pine (Pinus sylvestris L.) seedlings. Eur J Forest Res 132:481–496
Türkiye Meteorological Service (2020) General Directorate of Türkiye Meteorology Affairs
Vavrus S, Walsh JE, Chapman WL, Portis D (2006) The behavior of extreme cold air outbreaks under greenhouse warming. Int J Climatol 26:1133–1147
Acknowledgements
We thank the General Directorate of Forestry and all the individuals linked to the establishment and maintenance of the provenance trials in Türkiye.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Contributions
Bİ and AS designed and conducted the field experiment, CAGB ran the data analysis and led discussion, and all authors wrote the paper and discussed the results.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Communicated by Martin Ehbrecht.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
İmal, B., Semerci, A. & Gonzalez-Benecke, C.A. Intraspecific variability in cold hardiness of Taurus cedar (Cedrus libani A. Rich.) in Türkiye. Eur J Forest Res 143, 727–737 (2024). https://doi.org/10.1007/s10342-023-01648-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10342-023-01648-8