Abstract
Key message
We examined the leaf structure of two basal Cupressus species with a distinct leaf dimorphism. Some foliar features are regarded as ancestral and it is suggested that drought adaptation was one of the important ecological drivers in the evolution of the Cupressus genus.
Abstract
Leaf morphology and anatomy of two Cupressus species, C. nootkatensis and C. vietnamensis, were investigated with classical paraffin technique and scanning electron microscopy (SEM). Like all Cupressus species these two are characterised by a dramatic change in the foliage. Juveniles have needle leaves first before they change abruptly to the mature scale leaf type. In C. vietnamensis, needle-leaved shoots occur next to scale-leaved ones even on mature trees, which is unique among today´s Cupressus species. Adults of C. nootkatensis develop only scale leaves throughout. In both taxa, the scale leaves show a distinct dimorphism between lateral and facial leaves, which are arranged in a flat spray; the foliate shoots are two-dimensionally flattened. These scale leaves show several xeromorphic features; e.g. strongly reduced leaf size, stomata with high, collar-like Florin rings, the presence of a distinct hypodermis as a continuous layer and well-developed transfusion tissue. The needle leaf type is found in Cunninghamia which is the basal member of the Cupressaceae and so is regarded as the ancestral condition and scale leaves as a derived one. Scale leaves are found in all the members of the cupressoid clade even within the basal taxa from mesic habitats. However scale leaves are a preadaptation to survival under xeric conditions and they are likely an evolutionary driver of the radiation of Cupressus into arid environments, as has also been the case in genera such a Callitris.
Similar content being viewed by others
Introduction
In most gymnosperms, leaves are the major structure for photosynthesis. Only in a few taxa are the leaves strongly reduced so that photosynthesis is restricted to green stems, as is the case in most Ephedra species (Ephedraceae, Gnetales). In this genus, the leaves are reduced to tiny, membranous chlorophyll-free scales (e.g., Thompson 1912; Voth 1934; Cutler 1939; Inamdar and Bhatt 1971; Foster 1972; Price 1996; Freitag and Maier-Stolte 2003; Ickert-Bond and Wojciechowski 2004; Dörken 2014). In others, e.g. in all Phyllocladus species (Podocarpaceae, Coniferales), photosynthesis is restricted to highly complex phylloclades, which represent a fusion product of lateral shoot axes and inserted leaves (Keng H 1963a, b, 1973, 1974, 1978; Keng RS 1979; Quinn 1987; Tomlinson et al. 1989a, b; Wagstaff 2004; Dörken et al. 2021). A similar situation occurs in Sciadopitys (Sciadopityaceae, Coniferales), whose “double needles” represent a fusion product of two leaves and the short shoot axis (Dörken and Stützel 2011).
Leaves in conifers (Coniferales) show the greatest diversity among the recent gymnosperms and various leaf shapes can be found (Feustel 1921; Coulter and Chamberlain 1928; Krüssmann 1983; Eckenwalder 2009; Farjon 2005, 2010). The majority of taxa have single-veined needle leaves, with leaves that vary strongly in size depending on the species. Additionally, there are numerous scale-leaved taxa with strongly reduced leaves, e.g. numerous Cupressaceae and Podocarpaceae. However, juveniles of these groups have needle leaves, with a distinct change in the foliage in adults (Fig. 1) (Feustel 1921; de Laubenfels 1953; Langner 1963; Napp-Zinn 1966; Little 2006; Dörken 2013). While age is important light and water conditions may also have a strong influence on the leaf arrangement, shape and physiology (Dörken and Lepetit 2018 (Abies, Pinaceae); Zhang et al. 2021 (Juniperus, Cupressaceae). In some tropical conifers, large multi-veined foliar leaves are developed as is the case in, for example, Nageia (Podocarpaceae) or Agathis (Araucariaceae) (Feustel 1921; Page 1990; Dörken and Nimsch 2019). It is likely that such broad-leaved species are relatively recently evolved from smaller-leaved ancestors in response to competition with broad-leaved angiosperms in the Tertiary (e.g., see Chen et al. 2022 for podocarp phylogeny). In most taxa, there is just a single leaf type, either needle leaves, scale leaves or broad foliar leaves.
However, some conifers have a distinct leaf dimorphism, which can be classified into two major groups (1) Different leaf types e.g. scale and needle leaves, are developed on the same tree or even on the same branch; (2) One leaf type is developed but the leaves vary strongly in size and shape; e.g. scale leaves that can be divided into lateral and facial leaves. The first type can be found in some Cupressaceae (e.g. Juniperus phoenicea L. or Callitris macleayana (F. Muell.) F. Muell.) or in some Podocarpaceae (e.g. Dacrycarpus, Falcatifolium, Halocarpus and Manoao) (Eckenwalder 2009; Farjon 2010; Dörken et al. 2019a; Dörken and Nimsch 2019). Species belonging to the genus Pinus are exceptional because as adults the long shoot leaves are just rudimentary membranous scales that have no chlorophyll and photosynthesis is restricted to the needle-shaped short shoot leaves (Dörken et al. 2010a, b). This is remarkable because on juveniles, long shoot leaves are also photosynthetic needle leaves. The change in the long-shoot foliage from needle to scaly long-shoot leaves takes place when the first short shoots are developed (Powell 2009; Dörken et al 2010a, b; Dörken and Nimsch 2021a). The second type of leaf dimorphism is quite common for scale-leaved Cupressaceae e.g., Austrocedrus, Calocedrus, Chamaecyparis, Platycladus, Thuja and Papuacedrus. Cupressus nootkatensis and C. vietnamensis are the only representatives of the genus Cupressus having such a distinct differentiation into lateral and facial leaf. The majority of Cupressus species just have a uniform type of scale leaf (Eckenwalder 2009; Farjon 2005, 2010; Dörken and Nimsch 2019). Cupressus vietnamensis (Farjon & T.H. Nguyên) Silba is in several respects quite remarkable, because: (1) A distinct dimorphism between needle and scale leaves also occurs on mature individuals; (2) The scale leaves also show lateral and facial leaves; (3) The needle leaves are arranged in tetramerous whorls and the scale leaves are decussate.
In this study, we investigate the morphology and anatomy of leaves in Cupressus nootkatensis and Cupressus vietnamensis. The study aims to give new insights into the structural differences between the different leaf types and also the ecological reasons leading to leaf dimorphism. C. nootkatensis and C. vietnamensis are interesting research objects, not just because of their leaf dimorphism. They are regarded as representing the most basal taxa within Cupressus and occur in completely different environments. C. nootkatensis is a northwestern North American species native to Pacific coast regions from northern California to southern Alaska. It occurs on soils from rocky outcrops to peats, from sea level up to 2300 m (Farjon 2005, 2010; Eckenwalder 2009). Cupressus vietnamensis is native to subtropical cloud forests and occurs on limestone in the northern Vietnam Bat Dai Son Mountains (Ha Giang Province) on rocky slopes or strongly eroded ridge tops covered with a thin soil layer at about 1000–1300 m above sea level (Farjon 2005, 2010; Rushforth 2007; Eckenwalder 2009). Both species occur in relatively mesic environments with > 1500 mm rainfall/annum.
Material and methods
Origin of plant material
All plant material was collected in the Botanic Garden of the University of Konstanz, Konstanz, Germany. Due to low winter hardiness, C. vietnamensis is cultivated as a pot plant in a temperate house.
Methods
Freshly collected material was photographed and then fixed in FAA (100 ml FAA = 90 ml ethanol 70% + 5 ml acetic acid 96% + 5 ml formaldehyde solution 37%) before being stored in 70% ethanol.
The leaf and stem anatomy were studied from representative tissues from one plant in serial sections using the paraffin technique and subsequent astrablue/safranin staining (Gerlach 1984). Sections were prepared with a thickness between 12-16 µm. Several leaves were used because transverse and longitudinal sections and SEM samples were needed. Determinations of the cuticle thickness and the size of the epidermal cells were carried out with the Keyence VHX-500F software tool box. For each feature, 10 measurements were made on each of 10 leaves and maximum and minimum values reported.
For scanning electron microscopy (SEM) analysis, the FAA-material was dehydrated in formaldehyde dimethyl acetal (FDA) for 24 h (Gerstberger and Leins 1978) and later critical point dried. Sputter coating was done with a Sputter Coater SCD 50 Bal-tec (Balzers). The specimens were examined with an Auriga Zeiss TM.
Macrophotography used a digital camera (Canon PowerShot IS2) and microphotography a digital microscope (Keyence VHX 500F) equipped with a high-precision VH mounting stand with X–Y stage and bright-field illumination (Keyence VH-S5).
Special terms
Lateral and facial leaves: In this study, we describe a contrast between lateral and facial leaves for a scale-leaved decussate foliage arranged in a flat spray of the shoots with a distinct difference in the size and shape between leaves of the alternating pairs.
Taxonomic note
The taxonomic assignment of these two species has been the subject of considerable discussion. The Vietnamese Golden Cypress (here called Cupressus vietnamensis) was discovered in 1999 by Vietnamese botanists (Maerki 2017). Because of several morphological features such as its small seed cones and the distinct leaf dimorphism it was described as a new cupressaceous genus—Xanthocyparis (Farjon et al. 2002). Due to some structural similarities, the Nootka Cypress was also assigned to the new genus and was treated as X. nootkatensis. Little et al. (2004) remarked that the genus name Callitropsis (first mentioned by Oersted 1864) had priority over the name Xanthocyparis and treated both Xanthocyparis species as Callitropsis nootkatensis Oersted and Callitropsis vietnamensis (Farjon and Hiep) D. P. Little. The phylogram from a more recent comprehensive study of the phylogeny of gymnosperms (Stull et al. 2021) shows the relationship between Xanthocyparis, Callitropsis, Hesperocyparis, Cupressus and Juniperus and recognizes all five genera as monophyletic. However, the point at which the taxa are recognized as monophyletic can be a matter of opinion. The group of four genera (Xanthocyparis, Callitropsis, Hesperocyparis and Cupressus) can also be considered as monophyletic and sister to Juniperus as shown in the phylogram of Stull et al. (2021, supp Fig. 13) although there is one juniper species (J. maritima R.P.Adams) nested with Hesperocyparis in that figure. We prefer here to retain the broader circumscription of the genera as Cupressus due to morphological features that have been described elsewhere. Compared to the majority of the Cupressus species, the Nootka Cypress seed cones are just smaller and have fewer ovules. Thus, Jagel and Stützel (2001) supported the treatment of the Nootka Cypress as C. nootkatensis under which it was described by D. Don in 1824 as it was also accepted by Frankis (1993) and Silba (2005). In a later study carried out by Dörken et al. (2017) dealing with the ontogeny and structure of the Vietnamese Golden Cypress seed cones it was shown that there is no reliable argument that could justify a separation of the species from Cupressus. In Yang et al. (2012), both Cupressus vietnamensis and Cupressus nootkatensis are placed at the base of the new world Cupressus species. This fits well with the results of Dörken et al. (2017), who described both taxa as showing several of the ancestral features of the genus Cupressus. Biogeographically the placing of C. vietnamensis as sister to the old world cypresses (both are in Europe or Asia) and C. nootkatensis as sister to the new world cypresses (both on the American continent) in the Stull et al. (2021) phylogram makes sense.
Results
Cupressus nootkatensis
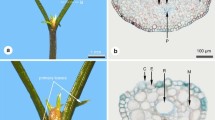
Lateral branchlets are plagiotropic to downward, so that there is a light exposed upper and a shaded lower shoot surface (Fig. 1a). On adult trees, there are only scale leaves (Figs. 2a, 3a). On lateral branchlets there is a slight difference between leaves as lateral leaves are keeled to folded while facial leaves are somewhat flattened (Fig. 2a). Lateral leaves are 2.3–3.2 mm long and 1.0–1.5 mm wide, facial leaves are 2.8–3.6 mm long and 0.6–1.0 mm wide. The tips of the facial leaves overtop those of the lateral ones (Figs. 2a, 3a). The adaxial leaf surfaces are strongly appressed to the shoot axis so that the foliate shoots are flattened (Fig. 1a). Both lateral and facial leaves terminate in a distinct hyaline tip (Fig. 3b). Leaves on the upper, light-exposed shoot surface are dark green (Fig. 2a), while those of the lower shoot surface are light green (Fig. 2a). Conspicuous wax markings are absent but leaves on both shoot surfaces are covered with a distinct cuticle, thicker on the shaded surface than the light exposed one (Table 1).
Cupressus foliage a C. nootkatensis mature foliage, in facial leaves of both surfaces there is a central depression below the tip, which is formed by the non-secreting resin duct (arrows); b, c C. vietnamensis b needle leaves: c adult scale leaves, lower surface slightly less light exposed, paler green
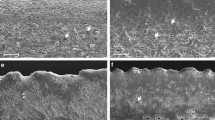
Cupressus nootkatensis, morphology of scale leaves (SEM-images); a lower surface of a shoot axis showing the stomatal distribution; b detail of a lateral leaf showing numerous stomata developed in the shaded part which is located towards the facial leaf; c stomatal field; d stoma with a high Florin ring (arrow)
Stomata are developed mostly on the shaded leaf surfaces. The majority is developed on leaf surfaces of the lower side of the shoot axis (Fig. 3a) and on shaded parts of lateral leaves Fig. 3b), which are located towards the facial leaves. In the stomatal fields they are irregularly arranged without any special orientation of the stomatal pore (Fig. 3c). The stomata are surrounded by a high, collar-like Florin ring (Fig. 3d), which is 38–46 µm long and 25–35 µm wide. The stomatal pore is 13–18 µm long. There is a large respiratory chamber (Fig. 4c). Below the epidermis, there is a well-developed hypodermis consisting of strongly lignified cells (Fig. 4b). The leaves are inverse-bifacial. The mesophyll is dimorphic with palisade parenchyma located towards all light-exposed parts of the leaves, while spongy parenchyma is located towards all shaded parts (Fig. 4a) and has a sparse cell density. The leaf is supplied with a single collateral vascular bundle strand, with xylem located towards adaxial and phloem towards abaxial surfaces (Fig. 4d). A distinct bundle sheath is absent (Fig. 4d). There is a large accessory transfusion tissue consisting of numerous transfusion tracheids (Fig. 4d). Below the vascular bundle there is one resin duct showing a well-developed sclerenchymatic, multi-layered sheath (Fig. 4e). This feature is developed in both leaf types, lateral and facial leaves. Due to the fact that the resin ducts are not located at the same level, it is impossible to observe them all in one section and to illustrate them in a single image. They are non-secreting and on facial leaves visible as a small, longitudinal depression below the tip (Fig. 2a).
Cupressus nootkatensis, anatomy of scale leaves; a cross section of a foliate shoot; all light exposed upper leaf surfaces with sun leaf character, the ones of the lower shaded side with shade leaf character; b leaf cross section showing the palisade of the abaxial surface; c stomata in more or less the same plane as the epidermis; d vascular bundle of a facial leaf with numerous accessory transfusion tracheids; e resin duct with sclerified multilayered sheath; X xylem, P phloem
Cupressus vietnamensis
There is a distinct leaf dimorphism with needle and scale leaves even on mature individuals. The needle-leaved shoots are in various orientations and the lateral-scale leaved shoots are ascending or orthotropic (Fig. 1b), so leaves of both shoot surfaces have more or less similar light exposure.
Needle leaves
On mature individuals, needle-leaved shoots can be found in different regions of the crown (Fig. 1b). Leaves are arranged in whorls of four, and eight orthostichies are formed. The needle leaves are bifacial structures showing an upper light exposed and a lower shaded surface (Fig. 2b). The adaxial side is dark green, and the abaxial one light green with two, whitish longitudinal bands marking the stomatal fields. A well-developed midrib occurs on both sides of the leaf. The leaves are 16–23 mm long and 1.3–2.4 mm wide. The broadest part of the leaf is the lowest fourth. Stomata are mostly abaxial in 20–26 longitudinal rows and are divided by the raised midrib into two bands (Figs. 2b, 5a). Adaxial stomata are sparse and many leaves were found without any. There is no distinct Florin ring in the majority of stomata; only a few show a weakly developed, flat one (Fig. 5b–d). The stomatal pore is 22–28 µm long and 7–10 µm wide underlain by a large respiratory chamber (Fig. 6e).
Cupressus vietnamensis, anatomy of needle leaves; a cross section of a bifacial needle leaf; palisade parenchyma is placed towards the light exposed adaxial surfaces; a hypodermis is only developed above and below the vascular bundle and at the leaf margin; b detail of the leaf margin; c leaf cross section of the adaxial surface; d vascular bundle with some accessory transfusion tracheids; below the bundle strand there is a single resin duct with parenchymatic single-layered sheath; e stomata and epidermis in more or less the same plane; X xylem, P phloem, TT transfusion tracheids
The epidermis of both surfaces is covered with a distinct cuticle (Table 1, Fig. 6b, c, e). A hypodermis is discontinuous, being found only below the vascular bundle strand and at the lateral leaf margins, consisting of one or two rows of strongly lignified cells (Fig. 6b). The mesophyll is dimorphic with well-developed palisade parenchyma towards the adaxial side and spongy parenchyma towards the abaxial one (Fig. 6a). There is a single collateral vascular bundle, with xylem towards the adaxial side and phloem towards the abaxial one (Fig. 6a, d). A distinct bundle sheath is absent. There are some accessory transfusion tracheids close to the vascular bundle (Fig. 6d). A resin duct with a single layer of parenchymatic cells is located below the vascular strand (Fig. 6a).
Scale leaves
Despite the distinct leaf dimorphism, on mature trees the majority of shoots are scale-leaved (Fig. 1a). There are no transitional leaves leading from needle to scale leaves. The change in the foliage is abrupt. The phyllotaxis on scale-leaved shoots is decussate, forming four orthostichies (Figs. 2c, 8a). The scale leaves can be categorized into keeled lateral and flattened facial leaves (Fig. 2c). The lateral ones are 2.7–4.0 mm long and 0.7–1.0 mm wide. The midrib on facial leaves is raised and roundish. Facial leaves are 1.2–3.0 mm long and 1.0–1.4 mm wide. The position of the leaf tips is various, facial leaves are in the same plane as the tips of the lateral ones, or they are longer or shorter (Figs. 2c, 7a). The basal adaxial leaf surfaces of the lateral leaves are strongly appressed to the shoot axis while the distal parts spread from the shoot axis (Fig. 7a, b). The entire adaxial surface of facial leaves is strongly appressed to the shoot axis so that scale-leaved shoots are flattened (Fig, 8a). Both lateral and facial leaves terminate in a distinct hyaline tip (Figs. 2c, 7a, b).
Cupressus vietnamensis, morphology of scale leaves (SEM-images); a lower surface of a shoot axis showing the stomatal distribution; b detail of a lateral leaf showing numerous stomata developed in the shaded part which is located towards the facial leaf; c stomatal field with blunt papillae; d stoma with a high Florin ring (arrow)
Despite the orthotropic short orientation there is usually minor deviation from the vertical plane so there is some development of more light exposed and more shaded sides of a shoot. This results in more light-exposed surfaces being darker green than the slightly shaded surfaces. The stomatal fields are recognisable by their conspicuous white wax markings. The leaves are amphistomatic with the majority of stomata developed on shaded leaf surfaces, mostly on the lower side of the shoot axis and on shaded parts of lateral leaves, which are located towards the facial leaves. In the stomatal fields, they are irregularly arranged, with no special orientation of the stomatal pore (Fig. 7c). All stomata are surrounded by a high, collar-like Florin ring (Fig. 7d), which is 33–46 µm long and 17–26 µm wide. The stomatal pore is 17–27 µm long with a large respiratory chamber below (Fig. 8c). The epidermis of both leaf surfaces is covered by a distinct cuticle and there are blunt papillae on the surface (Fig. 7c). There is a distinct and continuous hypodermis below the epidermis, which consists of one row of lignified cells with very thick walls (Fig. 8a, b). The mesophyll is dimorphic. Palisade parenchyma is located towards all light-exposed surfaces and spongy parenchyma with large intercellular spaces towards all shaded surfaces. The leaves are supplied by a single collateral vascular bundle. Xylem is located towards the adaxial and the phloem towards the abaxial side. There are accessory transfusion tracheids adjacent to the vascular bundle. A distinct bundle sheath is absent (Fig. 8d). In both leaf types, lateral and facial leaves, there is one resin duct with a single-layered parenchymatic sheath located below the vascular bundle (Fig. 8a, e). However, the resin ducts are not located all at the same level. Thus, it is impossible to hit them all in one section and to illustrate them in a single image.
Cupressus vietnamensis, anatomy of scale leaves; a cross section of a foliate shoot; facial and scale leaves are inverse bifacial, palisade parenchyma is placed towards all light exposed surfaces; b leaf tissues, showing the palisade of the abaxial surface; c stomata in more or less the same plane as the epidermis; d vascular bundle with some accessory transfusion tracheids; e resin duct with parenchymatic single-layered sheath; X xylem, P phloem, TT transfusion tracheids
Discussion
Leaf bifaciality
In the investigated species there are two types of leaves—needle and scale leaves. Bifaciality of two types was found: (1) that which affects the single leaf (needle and scale leaves of C. vietnamensis) (Figs. 1b, 5, 6, 7 and 8), (2) that which affects not the single leaf but the entire shoot axis (scale leaves of C. nootkatensis) (Figs. 1a, 3, 4). In both cases, palisade parenchyma occurs towards the light-exposed surfaces and spongy parenchyma towards the shaded ones. The needle leaves of C. vietnamensis (Figs. 1b, 2b, 5, 6) are flat structures with classical bifaciality as is typical for the majority of land plants. The situation in the C. vietnamensis scale leaves (Figs. 7, 8) is quite different. Despite some minor difference in the green colour intensity between the two sides of the shoot, due to their upright nature, palisade is developed on both sides of the lateral scale leaves but only on the abaxial side of the facial leaves, i.e., on the sun-exposed parts. A hypodermis occurs on the more light-exposed side but not on the shaded side, similar to scale leaves of C. nootkatensis where there is only hypodermis on the sun-exposed upper side of the shoot. Thus for both species, the morphologically adaxial surface is the functional lower surface and the morphologically abaxial side is the functional upper surface. In earlier work, this phenomenon was referred to as ‘inverse-dorsiventral’ (e.g., Imamura 1937; Fitting 1950; Napp-Zinn 1966) but more recent work almost always refers to it as ‘inverse-bifacial’. It occurs in many scale-leaved gymnosperms and angiosperms as well (Carlquist 1991; Kaplan 2001; Dörken and Parsons 2018). Thus, all light-exposed leaves have a sun leaf character, leaves on the lower shoot surface that of shade leaves. The bifaciality in C. nootkatensis (Fig. 8a) is more pronounced than in C. veitnamensis and does not only affect a single leaf but the entire foliate shoot due to its plagiotropic orientation.
This feature can also be found in other scale-leaved taxa with more or less plagiotropic or pendulous branchlets with a light-exposed and shaded surface (e.g. Chamaecyparis, Thuja and Thujopsis). In this case, bifaciality no longer corresponds to the morphologically ad- or abaxial side of a single leaf (e.g. Imamura 1937; Fitting 1950; Napp-Zinn 1966; Dörken 2013). This is environmentally controlled and generally reversible as earlier studies have shown (Imamura 1937; Fitting 1950; Napp-Zinn 1966; Dörken 2013). It is greatly influenced by light exposure, which can be proven in a simple experiment. When a plagiotropic shoot axis is brought into a new plane the formally shaded lower shoot surface becomes the new light-exposed one. Thus, the new foliage will develop palisade parenchyma exclusively towards light-exposed leaf surfaces and stomata will be developed on the new shaded surface. Thus, this special type of bifaciality affecting the entire foliate shoot and not just a single leaf is an anatomical response of the foliage to the angle of light exposure of the entire shoot and not to gravitational forces (Imamura 1937; Fitting 1950; Napp-Zinn 1966; Dörken 2013).
Evolutionary and ecological implications
All scale-leaved Cupressaceae are characterized by a dramatic change in the foliage from needle-leaved juveniles to scale-leaved adults (Fig. 1) (Feustel 1921; de Laubenfels 1953; Langner 1963; Napp-Zinn 1966; Little 2006; Dörken 2013). Mature individuals of C. vietnamensis also produce needle-leaved shoots in addition to scale-leaved ones. In all other Cupressus species mature individuals are exclusively scale leaved. The length of time before the foliage changes from juvenile needle leaf type to mature scale leaf varies greatly between species. Rushforth (2007) describes, in particular, the East Asian Cupressus species as taxa that retain the juvenile foliage for a decade or even longer so that juvenile and semi-adult/adult foliage occur even on the same branch as also found in Callitris macleayana (Dörken et al. 2019a).
Most Cupressus species are well adapted to drought. The majority of the New World Cupressus occur in relatively xeric habitats (Rushforth 1987; Eckenwalder 2009) but the Old World ones occurs over a broader climatic range including mesic environments (Little 2006). Thus, the leaf reduction from needle leaves to scale leaves might reflect an adaptation to drought and heat stress. There is a distinct difference between the foliage of most Cupressus and C. nootkatensis whose decussate foliage is arranged in a flat frond-like shoot axis. Due to the way the lateral and facial leaves are arranged the shoot axis is flattened which should maximise light capture of the scale leaves. This is advantageous for the species as it grows in a wet temperate environment where there is often considerable cloud cover producing diffuse, often low light conditions. In addition, its habitat experiences high snowfall so the microphyllous habit would aid snow shedding, helping to prevent tree damage due to heavy snow load.
Further correlation with the mesic habitat of the two species is emphasized by the comparatively large amount of intercellular space in the spongy mesophyll of both the needle and scale leaves. This is also the case in needle leaves of Cunninghamia (Dörken and Nimsch 2021b). This contrasts with the much denser cell packing in the very similar scale leaves of arid-adapted taxa such as Callitris (Dörken et al. 2019a). The strong leaf reduction of most Cupressus (and Callitris) forms foliate shoot axes representing green photosynthetic columns that show two great advantages over the needle leaves: (1) The transpiring surface is strongly reduced, which leads to a reduction in water loss via the photosynthetic surface; (2) The light interception of these photosynthetic columns at midday, which is mostly the hottest time of the day, is minimized because direct solar radiation strongly strikes only a minimum of the total leaf surface. Most leaf surfaces only receive diffuse radiation so heat load at the hottest time of the day is decreased. Light interception, however, is maximized early and late in the day when the temperatures are cooler and consequently water stress should be less severe (Falster and Westoby 2003; Yates et al. 2010; Dörken et al. 2023). Such reduction in leaf size and appression to the stem also occurs in numerous microphyllous angiosperms where leaf reduction is so strong, that photosynthetic columns are formed, such as for some Asteraceae and Myrtaceae due to microphyllous peltation and reflexion (Dörken et al. 2023).
In general, there is a strong tendency to form small leaves in dry conditions (Carlson et al. 2010; Dörken and Parsons 2017; Dörken et al. 2018, 2019a, b, 2020; Wang et al. 2022). Cunninghamia, with needle leaves, is basal to all cupressaceous taxa and occurs in mesophytic forest in Asia (Farjon 2005). Thus, the retention of needle leaves in C. vietnamensis seems a plesiomorphic trait and the reduction of needle leaves to scale leaves in the species involves characteristics that are xeromorphic or scleromorphic, such as a well-developed hypodermis or vascular bundles with large accessory transfusion tissue. The absence of a Florin ring in the majority of stomata on the needle leaves, which is typical for taxa from mesic sites, also fits well to a mesic origin for ancestral Cupressus.
While both species occur in generally mesic environments, the local sites in which they grow tend to have nutrient-poor soils (Farjon 2005) and may suffer from water deficit due to the skeletal soils (for C. vietnamensis) or freezing winter temperatures (for C. nootkatensis). As noted above for C. nootkatensis the microphyllous foliage in combination with pendulant, drooping branches may help to shed snow more easily from the crown than species with larger needle leaves and plagiotropic branchlets, so that mechanical damages caused by heavy snow loads would be reduced.
On the one hand, a microphyllous foliage reduces the heat load and transpiration in times of drought, and while this is not likely to be relevant to either species, on the other hand, it also facilitates nutrient uptake from infertile soils during cooler and wetter conditions (Yates et al. 2010). Thus, different selective forces can lead to quite similar adaptations to particular environments. In the case of Cupressus, where numerous species not only occur in hot and dry climates but also on sandy and rocky soils, the adaptation to nutrient-poor soils could also have played an important role in evolution and speciation. It remains open if the leaf reduction in Cupressus was initially a xeromorphic or scleromorphic adaptation. However, the tendency of species in the most mesic habitats to retain juvenile foliage for longer than species from xeric habitats tends to indicate water deficit is the more pressing evolutionary force (e.g., New Caledonian Callitris species, Farjon (2005), Callitris macleayana Dörken et al. (2019a)). Despite what the initial driver for the evolution of scale leaves in Cupressus was, the microphyllous foliage was an important preadaptation to subsequently conquer habitats with either xeric or nutrient-poor conditions. This is very similar to what has been suggested from physiological studies of the very similarly structured Callitris where evolution in that genus has been attributed to increasing aridity from the Oligocene (Larter et al. 2017). Thus, in many of today´s Cupressus species the scale leaves are important structural traits that benefit the species in environments that are characterized by both water and nutrient deficit.
Data availability
All data in this paper are presented in the tables and figures.
References
Carlquist S (1991) Leaf anatomy of Bruniaceae: ecological, systematic and phylogenetic aspects. Bot J Linn Soc 107(1):1–34. https://doi.org/10.1111/j.1095-8339.1991.tb00212.x
Carlson JE, Holsinger KE, Prunier R (2010) Plant responses to climate in the Cape Floristic region of South Africa: evidence for adaptive differentiation in the Proteaceae. Evolution 65:108–124. https://doi.org/10.1111/j.1558-5646.2010.01131.x
Chen L, Jin WT, Liu XQ, Wang XQ (2022) New insights into the phylogeny and evolution of Podocarpaceae inferred from transcriptomic data. Mol Phylo Evol 166:107341. https://doi.org/10.1016/j.ympev.2021.107341
Coulter JM, Chamberlain CJ (1928) Morphology of gymnosperms, 4th edn. University of Chicago Press, Chicago
Cutler HC (1939) Monograph of the North American species of the genus Ephedra. Ann Mo Bot Gard 26:373–428
De Laubenfels DJ (1953) The external morphology of coniferous leaves. Phytomorph 3(1–2):1–20
Dörken VM (2013) Leaf dimorphism in Thuja plicata and Platycladus orientalis (thujoid Cupressaceae s. str., Coniferales): the changes in morphology and anatomy from juvenile needle leaves to mature scale leaves. Plant Syst Evol 299:1991–2001. https://doi.org/10.1007/s00606-013-0853-3
Dörken VM (2014) Leaf-morphology and leaf-anatomy in Ephedra altissima Desf. (Ephedraceae, Gnetales) and their evolutionary relevance. Feddes Repert 123:243–255. https://doi.org/10.1002/fedr.201200020
Dörken VM, Lepetit B (2018) Morpho-anatomical and physiological differences between sun and shade leaves in Abies alba Mill. (Pinaceae, Coniferales): a combined approach. Plant Cell Environ 41:1683–1697. https://doi.org/10.1111/pce.13213
Dörken VM, Nimsch H (2019) Morphology and Identification of Coniferous Genera. Verlag Kessel, Remagen-Oberwinter
Dörken VM, Nimsch H (2021a) Pinus monophylla Torr. et Frém. (Pinaceae)—Eine bemerkenswerte Kiefer aus den semiariden Gebirgsregionen der SW-USA und NW-Mexikos. Schweiz Beitr Dendrol 71–72:46–54
Dörken VM, Nimsch H (2021b) Spießtannen (Cunninghamia, Cupressaceae s. l.)—eine Gattungsübersicht. Schweiz Beitr Dendrol 71–72:55–66
Dörken VM, Parsons RF (2017) Morpho-anatomical studies on the leaf reduction in Casuarina (Casuarinaceae): the ecology of xeromorphy. Trees 31:1165–1177. https://doi.org/10.1007/s00468-017-1535-5
Dörken VM, Parsons RF (2018) The foliar change in two species of Melaleuca (Myrtaceae): a morpho-anatomic and ontogenetic approach. Trees 32(4):1013–1028. https://doi.org/10.1007/s00468-018-1692-1
Dörken VM, Stützel T (2011) Morphology and anatomy of anomalous cladodes in Sciadopitys verticillata Siebold & Zucc. (Sciadopityaceae). Trees 25:199–213. https://doi.org/10.1007/s00468-010-0498-6
Dörken VM, Stephan G, Stüzel Th (2010a) Morphology and anatomy of anomalous short shoots in Pinus (Pinaceae) and their evolutionary meaning. Feddes Repert 121:133–150. https://doi.org/10.1002/fedr.201000006
Dörken VM, Stephan G, Stützel Th (2010b) Morphology and anatomy of anomalous short shoots in Pinus (Pinaceae) and their evolutionary meaning. Feddes Repert 121:133–150. https://doi.org/10.1002/fedr.201000006
Dörken VM, Nimsch H, Jagel A (2017) Morphology, anatomy and morphogenesis of seed cones of Cupressus vietnamensis (Cupressaceae) and the taxonomic and systematic implications. Flora 230:47–56. https://doi.org/10.1016/j.flora.2017.03.008
Dörken VM, Parsons RF, Ladd PG (2018) The foliar change from seedlings to adults in Allocasuarina (Casuarinaceae): the evolutionary and ecological aspects of leaf reduction, xeromorphy and scleromorphy. Feddes Repert 129:193–222. https://doi.org/10.1002/fedr.201800004
Dörken VM, Ladd PG, Parsons RF (2019a) The foliar characters in Callitris (Callitroideae, Cupressaceae s. str.) and their evolutionary and ecological significance. Feddes Repert 130:247–271. https://doi.org/10.1002/fedr.201800033
Dörken VM, Ladd PG, Parsons RF (2019b) Foliar ontogeny in Gymnostoma deplancheanum and its evolutionary and ecological significance for scleromorphy and xeromorphy in Casuarinaceae (Fagales). Trees 33:653–668. https://doi.org/10.1007/s00468-018-1806-9
Dörken VM, Ladd PG, Parsons RF (2020) Anatomical aspects of xeromorphy in arid-adapted plants of Australia. Aust J Bot 68:245–266. https://doi.org/10.1071/BT19073
Dörken VM, Hill RS, Jordan GJ, Parsons RF (2021) Evolutionary and ecological significance of photosynthetic organs in Phyllocladus (Podocarpaceae). Bot J Linn Soc 196:343–363. https://doi.org/10.1093/botlinnean/boaa106
Dörken VM, Ladd PG, Parsons RF (2023) Convergent morphology and anatomy in the microphyllous leaves of selected heathland Myrtaceae and Asteraceae. Trees (in press)
Eckenwalder JE (2009) Conifers of the world. Timber Press, Portland
Falster DS, Westoby M (2003) Leaf size and angle vary widely across species: what consequences for light interception? New Phytol 158:509–525. https://doi.org/10.1046/j.1469-8137.2003.00765.x
Farjon A (2005) A monograph of Cupressaceae and Sciadopitys. Royal Botanic Gardens Kew, Richmond
Farjon A (2010) A handbook of the world´s conifers. Brill, Boston
Farjon A, Hiep NT, Harder DK, Loc PK, Averyanov L (2002) A new genus and species in Cupressaceae (Coniferales) from northern Vietnam, Xanthocyparis vietnamensis. Novon 12:179–189. https://doi.org/10.2307/3392953
Feustel H (1921) Anatomie und Biologie der Gymnospermenblätter. Beihefte Bot Centralbl 38(2):177–257
Fitting H (1950) Weitere Beobachtungen über die Induktion der Dorsiventralität in den blattartigen Zweigsystemen von Cupressaceen. Planta 37:676–696
Foster A (1972) Venation patterns in leaves of Ephedra. J Arnold Arbor 53(3):364–385
Frankis MP (1993) Nootka Cypress: Chamaecyparis or Cupressus? Conifer Soc Australia Newsl 12:9–10
Freitag H, Maier-Stolte M (2003) The genus Ephedra in NE Tropical Africa. Kew Bull 58:415–426
Gerlach D (1984) Botanische Mikrotomtechnik, eine Einführung, 2nd edn. Thieme, Stuttgart
Gerstberger P, Leins P (1978) Rasterelektronenmikroskopische Untersuchungen an Blütenknospen von Physalis philadelphia (Solanaceae). Ber Dtsch Bot Ges 91:381–387. https://doi.org/10.1111/j.1438-8677.1978.tb03660.x
Ickert-Bond SM, Wojciechowski MF (2004) Phylogenetic relationships in Ephedra (Gnetales): Evidence from nuclear and chloroplast DNA sequence data. Syst Bot 29(4):834–849. https://doi.org/10.1600/0363644042451143
Imamura SL (1937) Über die aitiogene Dorsiventralität der Assimilationsorgane bei höheren Pflanzen. Bot Mag 61(606):308–316
Inamdar JA, Bhatt D (1971) Epidermal structure and ontogeny of stomata in vegetative and reproductive organs of Ephedra and Gnetum. Ann Bot 36:1041–1046. https://doi.org/10.1093/oxfordjournals.aob.a084647
Jagel A, Stützel T (2001) Zur Abgrenzung von Chamaecyparis Spach und Cupressus L. (Cupressaceae) und die systematische Stellung von Cupressus nootkatensis D. Don [= Chamaecyparis nootkatensis (D. Don) Spach]. Feddes Repert 112(3–4):179–229. https://doi.org/10.1002/fedr.20011120304
Kaplan DR (2001) Fundamental concepts of leaf morphology and morphogenesis: a contribution to the interpretation of molecular genetic mutants. Int J Plant Sci 162(3):465–474. https://doi.org/10.1086/320135
Keng H (1963a) Aspects of morphology of Phyllocladus hypophyllus. Ann Bot 27:69–78. https://doi.org/10.1093/oxfordjournals.aob.a083836
Keng H (1963b) Phyllocladus hypophyllus Hook. f. Gardens Bull Singap 20:123–126
Keng H (1973) On the family Phyllocladaceae. Taiwania 18:142–145
Keng H (1974) The phylloclade of Phyllocladus and its possible bearing on the branch systems of Progymnosperms. Ann Bot 38:757–764. https://doi.org/10.1093/oxfordjournals.aob.a084864
Keng H (1978) The genus Phyllocladus (Phyllocladaceae). J Arnold Arbor 59:249–273
Keng RS (1979) Comparative anatomy of the species of Phyllocladus (Coniferae), a preliminary study. J Taiwan Mus 32:221–306. https://doi.org/10.6532/QJTM.197912_32(3_4).0004
Krüssmann G (1983) Handbuch der Nadelgehölze, 2nd edn. Parey, Berlin
Langner W (1963) Die Entstehung sogenannter Jugendformen bei Chamaecyparis. Silv Gen 13(3):57–63
Larter M, Pfautsch S, Domec J-C, Trueba S, Nagalingum N, Delzon S (2017) Aridity drove the evolution of extreme embolism resistance and the radiation of conifer genus Callitris. New Phytol 215:97–112. https://doi.org/10.1111/nph.14545
Little DP (2006) Evolution and circumscription of the true cypresses (Cupressaceae: Cupressus). Syst Bot 31(3):461–480. https://doi.org/10.1600/036364406778388638
Little DP, Schwarzbach A, Adams RP, Hsieh CF (2004) The circumscription and phylogenetic relationship of Callitropsis and the newly described genus Xanthocyparis (Cupressaceae). Amer J Bot 91(11):1872–1881. https://doi.org/10.3732/ajb.91.11.1872
Maerki D (2017) Recent developments in the taxonomy of the genus Cupressus and consequences for their conservation. Bull Cupressus Conservation Proj 6(1):3–24
Napp-Zinn K (1966) Anatomie des Blattes. I. Blattanatomie der Gymnospermen. In: Zimmermann W, Ozenda P, Wulff HD (eds) Handbuch der Pflanzenanatomie, vol 8. Bornträger, Berlin, pp 111–121
Oersted AS (1864) Bidrag til Naaletræernes Morphologi. Videnskabelige Meddelelser Fra Den Naturhistorisk Forening i Kjobenhavn Series 2(6):1–36
Page CN (1990) Coniferophytina (Conifers and Ginkgoids). In: Kubitzki K (ed) The families and genera of vascular plants, vol 1. Springer, Heidelberg, pp 282–361
Powell GR (2009) Lives of conifers—a comparative account of the coniferous trees. The Johns Hopkins University Press, Baltimore
Price RA (1996) Systematics of gnetales: a review of morphological and molecular evidence. Int J Plant Sci 157(6 Suppl.):40–49
Quinn CJ (1987) The Phyllocladaceae Keng—a critique. Taxon 36:559–565. https://doi.org/10.2307/1221846
Rushforth K (1987) Conifers. Christopher Helm, London
Rushforth K (2007) Notes on the Cupressaceae in Vietnam. Vietnam J Biol 29(1):32–39
Silba J (2005) A monograph of the genus Cupressus L. in the twenty-first century. J Int Conifer Preserv Soc 12:31–103
Stull GW, Qu XJ, Parins-Fukuchi C, Yang YY, Yang JB, Yang ZY, Hu Y, Soltis PS, Soltis DE, Li DZ, Smith SA, Yi TS (2021) Gene duplications and phylogenomic conflict underlie major pulses of phenotypic evolution in gymnosperms. Nat Plants 7(8):1015–1025
Thompson WP (1912) The anatomy and relationships of Gnetales. I. The genus Ephedra. Ann Bot 27:1077–1104
Tomlinson PB, Takaso T, Rattenbury JA (1989a) Cone and ovule ontogeny in Phyllocladus (Podocarpaceae). Bot J Linn Soc 99:209–221. https://doi.org/10.1111/j.1095-8339.1989.tb00400.x
Tomlinson PB, Takaso T, Rattenbury JA (1989b) Developmental shoot morphology in Phyllocladus (Podocarpaceae). Bot J Linn Soc 99:223–248. https://doi.org/10.1111/j.1095-8339.1989.tb00401.x
Voth PD (1934) A study of the vegetative phase of Ephedra. Bot Gaz 96(2):298–313
Wagstaff SJ (2004) Evolution and biogeography of the austral genus Phyllocladus (Podocarpaceae). J Biogeogr 32:1569–1577. https://doi.org/10.1111/j.1365-2699.2004.01066.x
Wang H, Wang R, Harrison SP, Prentice IC (2022) Leaf morphological traits as adaptations to multiple climatic gradients. J Ecol 110:1344–1355. https://doi.org/10.3389/fpls.2019.00058
Yang ZY, Ran JH, Wang XQ (2012) Three genome-based phylogeny of Cupressaceae s. l.: further evidence for the evolution of gymnosperms and Southern Hemisphere biogeography. Mol Phylogenet Evol 64:452–470. https://doi.org/10.1016/j.ympev.2012.05.004
Yates MJ, Verboom GA, Rebelo AG, Cramer MD (2010) Ecophysiological significance of leaf size variation in Proteaceae from the Cape Floristic region. Funct Ecol 24:485–492. https://doi.org/10.1111/j.1365-2435.2009.01678.x
Zhang JL, Li XG, Xu XH, Chen HP, Li YL, Guy RD (2021) Leaf morphology, photosynthesis and pigments change with age and light regime in savin juniper. Plant Biol 23(6):1097–1108. https://doi.org/10.1111/plb.13256
Acknowledgements
We thank the Botanic Garden of the University of Konstanz, (Konstanz, Germany) for producing the seedlings and Dr. Michael Laumann and Dr. Paavo Bergmann (Electron Microscopy Centre, Department of Biology, University of Konstanz, Germany) for technical support (paraffin technique and SEM). Furthermore, we thank the Cupressus Conservation Project for helpful comments and discussions on the systematics and taxonomy of the genus Cupressus. The comments of the two reviewers helped to improve the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. This research did not receive any specific funding.
Author information
Authors and Affiliations
Contributions
Writing—preparation of the original draft VMD, revision and editing: VMD, RP, PL. Conceptualization and planning of the project: VMD, RP, PL. Data analysis—VMD, RP, PL. Investigation and enquiry: VMD. Methods—sectioning and photography VMD. Material— collecting VMD.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Communicated by R. Guy .
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dörken, V.M., Ladd, P.G. & Parsons, R.F. Leaf dimorphism in Cupressus nootkatensis D. Don and Cupressus vietnamensis (Farjon & T.H. Nguyên) Silba (Cupressaceae) and its ecological and evolutionary significance. Trees 37, 1267–1279 (2023). https://doi.org/10.1007/s00468-023-02424-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-023-02424-2