Abstract
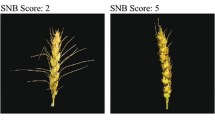
Septoria nodorum blotch (SNB) is a foliar disease of wheat caused by the necrotrophic fungal pathogen Parastagonospora nodorum. Research over the last two decades has shown that the wheat-P. nodorum pathosystem mostly follows an inverse gene-for-gene model. The fungus produces necrotrophic effectors (NEs) that interact with specific host gene products encoded by dominant sensitivity (S) genes. When a compatible interaction occurs, a ‘defense response’ in the host leads to programmed cell death thereby provided dead/dying cells from which the pathogen, being a necrotroph, can acquire nutrients allowing it to grow and sporulate. To date, nine S gene-NE interactions have been characterized in this pathosystem. Five NE-encoding genes, SnTox1, SnTox3, SnToxA, SnTox5, and SnTox267, have been cloned along with three host S genes, Tsn1, Snn1, and Snn3-D1. Studies have shown that P. nodorum hijacks multiple and diverse host targets to cause disease. SNB resistance is often quantitative in nature because multiple compatible interactions usually occur concomitantly. NE gene expression plays a key role in disease severity, and the effect of each compatible interaction can vary depending on the other existing compatible interactions. Numerous SNB-resistance QTL have been identified in addition to the known S genes, and more research is needed to understand the nature of these resistance loci. Marker-assisted elimination of S genes through conventional breeding practices and disruption of S genes using gene editing techniques are both effective strategies for the development of SNB-resistant wheat cultivars, which will become necessary as the global demand for sustenance grows.







Similar content being viewed by others
References
Abeysekara NS, Faris JD, Chao S, McClean PE, Friesen TL (2012) Whole-genome analysis of Stagonospora nodorum blotch resistance and validation of the SnTox4-Snn4 interaction in hexaploid wheat. Phytopathology 102:94–104
Abeysekara NS, Friesen TL, Keller B, Faris JD (2009) Identification and characterization of a novel host-toxin interaction in the wheat-Stagonospora nodorum pathosystem. Theor Appl Genet 120:117–126
Adhikari TB, Jackson EW, Gurung S, Hansen JM, Bonman JM (2011) Association mapping of quantitative resistance to Phaeosphaeria nodorum in spring wheat landraces from the USDA National Small Grains Collection. Phytopathology 101:1301–1310
Aguilar V, Stamp P, Winzeler M, Winzeler H, Schachermayr G, Keller B, Zanetti S, Messmer MM (2005) Inheritance of field resistance to Stagonospora nodorum leaf and glume blotch and correlations with other morphological traits in hexaploid wheat (Triticum aestivum L.). Theor Appl Genet 111:325–336
Arseniuk E, Czembor PC, Czaplicki A, Song Q, Cregan PB, Hoffman DL, Ueng PP (2004) QTL controlling partial resistance to Stagonospora nodorum leaf blotch in winter wheat cultivar Alba. Euphytica 137:225–231
Ballance GM, Lamari L, Bernier CC (1989) Purification and characterization of a host selective toxin from Pyrenophora tritici-repentis. Physiol Mol Plant P 35:203–213
Ballance GM, Lamari L, Kowatsch R, Bernier CC (1996) Cloning, expression and occurrence of the gene encoding the Ptr necrosis toxin from Pyrenophora tritici-repentis. Mol Plant Pathol http://w.bspp.org.uk/mppol/1996/1209ballance/
Ballini E, Tavaud M, Ducasse A, Sanchez D, Paux E, Kitt J, Charmet G, Audigeos D, Roumet P, David J, Morel J (2020) Genome wide association mapping for resistance to multiple fungal pathogens in a panel issued from a broad composite cross-population of tetraploid wheat Triticum turgidum. Euphytica 216:92
Bertucci M, Brown-Guedira G, Murphy JP, Cowger C (2014) Genes conferring sensitivity to Stagonospora nodorum necrotrophic effectors in Stagonospora nodorum blotch-susceptible U.S. wheat cultivars. Plant Dis 98:746–753
Breen S, Williams SJ, Winterberg B, Kobe B, Solomon PS (2016) Wheat PR-1 proteins are targeted by necrotrophic pathogen effector proteins. Plant J 88:13–25
Brueggeman R, Druka A, Nirmala J, Cavileer T, Drader T, Rostoks N, Mirlohi A, Beenypaul H, Gill U, Kudrna D, Whitelaw C, Killian A, Han F, Sun Y, Gill K, Steffenson B, Kleinhofs A (2008) The stem rust resistance gene Rpg5 encodes a protein with nucleotide-binding-site, leucine-rich, and protein kinase domains. Proc Natl Acad Sci 105:14970–14975
Ciuffetti LM, Manning VA, Pandelova I, Betts MF, Martinez JP (2010) Host-selective toxins, Ptr ToxA and Ptr ToxB, as necrotrophic effectors in the Pyrenophora tritici-repentis-wheat interaction. New Phytol 187:911–919
Ciufetti LM, Tuori RP (1999) Advances in the characterization of the Pyrenophora tritici-repentis-wheat interaction. Pytopathol 89:444–449
Ciuffetti LM, Tuori RP, Gaventa JM (1997) A single gene encodes a selective toxin causal to the development of tan spot of wheat. Plant Cell 9:135–144
Chu CG, Faris JD, Xu SS, Friesen TL (2010) Genetic analysis of disease susceptibility contributed by the compatible Tsn1-SnToxA and Snn1-SnTox1 interactions in the wheat-Stagonospora nodorum pathosystem. Theor Appl Genet 120:1451–1459
Cockram J, Scuderi A, Barber T, Furuki E, Gardner KA, Gosman N, Kowalczyk R, Phan HP, Rose GA, Tan KC, Oliver RP, Mackay IJ (2015) Fine-mapping the wheat Snn1 locus conferring sensitivity to the Parastagonospora nodorum necrotrophic effector SnTox1 using an eight founder multiparent advanced generation inter-cross population. G3-Genes Genom Genet 5:2257–2266
Couto D, Zipfel C (2016) Regulation of pattern recognition receptor signalling in plants. Nat Rev Immunol 16:537–552
Cowger C, Ward B, Brown-Guedira G, Brown JKM (2020) Role of effector-sensitivity gene interactions and durability of quantitative resistance to Septoria Nodorum Blotch in Eastern U.S. Wheat. Front Plant Sci. https://doi.org/10.3389/fpls.2020.00155
Crook AD, Friesen TL, Liu ZH, Ojimbo PS, Cowger C (2012) Novel necrotrophic effectors from Stagonospora nodorum and corresponding host sensitivities in winter wheat germplasm in the Southeastern United States. Phytopathology 102:498–505
Czembor PC, Arseniuk E, Czaplicki A, Song Q, Cregan PB, Ueng PP (2003) QTL mapping of partial resistance in winter wheat to Stagonospora nodorum blotch. Genome 46:546–554
Czembor PC, Arseniuk E, Radecka-Janusik M, Piechota U, Słowacki P (2019) Quantitative trait loci analysis of adult plant resistance to Parastagonospora nodorum blotch in winter wheat cv. Liwilla (Triticum aestivum L.). Eur J Plant Pathol 155:1001–1016
Day B, Henty JL, Porter KJ, Staiger CJ (2011) The pathogen-actin connection: a platform for defense signaling in plants. Annu Rev Phytopathol 49:483–506
Dickman MB, Park YK, Oltersdorf T, Li W, Clemente T, French R (2001) Abrogation of disease development in plants expressing animals antiapoptotic genes. Proc Natl Acad Sci USA 98:6957–6962
Downie RC, Bouvet L, Furuki E, Gosman N, Gardner KA, Mackay IJ, Mantello CC, Mellers G, Phan HTT, Rose GA, Tan KC, Oliver RP, Cackram J (2018) Assessing European wheat sensitivities to Parastagonospora nodorum necrotrophic effectors and fine-mapping the Snn3-B1 locus conferring sensitivity to the effector SnTox3. Front Plant Sci 9:881
Downie RC, Lin M, Corsi B, Ficke A, Lillemo M, Oliver RP, Phan HTT, Tan KC, Cockram J (2021) Septoria nodorum blotch of wheat: disease management and resistance breeding in the face of shifting disease dynamics and a changing environment. Phytopathology 111:906–920
Eyal Z (1987) The Septoria diseases of wheat: concepts and methods of disease management. CIMMYT, Mexico
Faris JD, Friesen TL (2009) Reevaluation of a tetraploid wheat population indicates that the Tsn1-ToxA interaction is the only factor governing Stagonospora nodorum blotch susceptibility. Phytopathology 99:906–912
Faris JD, Friesen TL (2020) Plant genes hijacked by necrotrophic fungal pathogens. Curr Opin Plant Biol 56:74–80
Faris JD, Anderson JA, Francl LJ, Jordahl JG (1996) Chromosomal location of a gene conditioning insensitivity in wheat to a necrosis-inducing culture filtrate from Pyrenophora tritici-repentis. Phytopathology 86:459–463
Faris JD, Liu Z, Xu SS (2013) Genetics of tan spot resistance in wheat. Theor Appl Genet 126:2197–2217
Faris JD, Zhang Z, Lu H, Lu Z, Reddy L, Cloutier S, Fellers JP, Meinhardt SW, Rasmussen JB, Xu SS, Oliver RP, Simons KJ, Friesen TL (2010) A unique wheat disease resistance-like gene governs effector-triggered susceptibility to necrotrophic pathogens. Proc Natl Acad Sci 107:13544–13549
Faris JD, Zhang Z, Rasmussen JB, Friesen TL (2011) Variable expression of the Stagonospora nodorum effector SnToxA among isolates correlated with levels of disease in wheat. Mol Plant Microbe In 24:1419–1426
Ficke A, Cowger C, Bergstrom G, Brodal G (2018) Understanding yield loss and pathogen biology to improve disease management: Septoria nodorum blotch-a case study in wheat. Plant Dis 102:696–707
Flor HH (1955) Host-parasite interaction in flax-rust-its genetics and other implications. Phytopathology 45:680–685
Flor HH (1956) Complementary genetic systems in flax and flax rust. Adv Genet 8:29–54
Francki MG (2013) Improving Stagonospora nodorum resistance in wheat: a review. Crop Sci 53:355–365
Francki MG, Shankar M, Walker E, Loughman R, Golzar H, Ohm H (2011) New quantitative trait loci in wheat for flag leaf resistance to Stagonospora nodorum blotch. Phytopathology 101:1278–1284
Francki MG, Walker E, McMullan CJ, Morris WG (2020) Multi-location evaluation of global wheat lines reveal multiple QTL for adult plant resistance to Septoria nodorum blotch (SNB) detected in specific environments and in response to different isolates. Front Plant Sci 11:771
Friesen TL, Faris JD (2010) Characterization of the wheat-Stagonospora nodorum system: what is the molecular basis of this quantitative necrotrophic disease interaction? Can J Plant Pathol 32:20–28
Friesen TL and Faris JD (2012) Characterization of plant-fungal interactions involving necrotrophic effector-producing plant pathogens. In: Bolton MD, and Thomma BPHJ (eds) Plant fungal pathogens: methods and protocols, methods in molecular biology, vol 835, https://doi.org/10.1007/978-1-61779-501-5_12
Friesen TL, Faris JD (2021) Characterization of effector-target interactions in necrotrophic pathosystems reveals trends and variation in host manipulation. Ann Rev Phytopathol 59:77–98
Friesen TL, Chu CG, Liu ZH, Xu SS, Halley S, Faris JD (2009) Host-selective toxins produced by Stagonospora nodorum confer disease susceptibility in adult wheat plants under field conditions. Theor Appl Genet 118:1489–1497
Friesen TL, Chu C, Xu SS, Faris JD (2012) SnTox5-Snn5: a novel Stagonospora nodorum effector-wheat gene interaction and its relationship with the SnToxA-Tsn1 and SnTox3-Snn3-B1 interactions. Mol Plant Pathol 13:1101–1109
Friesen TL, Holmes DJ, Bowden RL, Faris JD (2018) ToxA is present in U.S. Bipolaris sorokiniana population and is a significant virulence factor on wheat harboring Tsn1. Plant Dis 102:2446
Friesen TL, Meinhardt SW, Faris JD (2007) The Stagonospora nodorum-wheat pathosystem involves multiple proteinaceous host-selective toxins and corresponding host sensitivity genes that interact in an inverse gene-for-gene manner. Plant J 51:681–692
Friesen TL, Stukenbrock EH, Liu Z, Meinhardt S, Ling H, Faris JD, Rasmussen JB, Solomon PS, McDonald BA, Oliver RP (2006) Emergence of a new disease as a result of interspecific virulence gene transfer. Nat Genet 38:953–956
Friesen TL, Zhang Z, Solomon PS, Oliver RP, Faris JD (2008) Characterization of the interaction of a novel Stagonospora nodorum host-selective toxin with a wheat susceptibility gene. Plant Physiol 146:682–693
Friskop A, Liu Z (2016) Fungal leaf spot diseases of wheat: tan spot, Septoria/Stagonospora nodorum blotch and Septoria tritici blotch. NDSU Extension Service, North Dakota State University
Gao Y, Faris JD, Liu Z, Kim YM, Syme RA, Oliver RP, Xu SS, Friesen TL (2015) Identification and characterization of the SnTox6-Snn6 interaction in the Parastagonospora nodorum-wheat pathosystem. Mol Plant Microbe In 28:615–625
Gurung S, Mamidi S, Bonman JM, Xiong M, Brown-Guedira G, Adhikari TB (2014) Genome-wide association study reveals novel quantitative loci associated with resistance to multiple leaf spot diseases of spring wheat. PLoS ONE 9:e108179
Haen KM, Lu HJ, Friesen TL, Faris JD (2004) Genomic targeting and high-resolution mapping of the Tsn1 gene in wheat. Crop Sci 44:951–962
Hafez M, Gourlie R, Despins T, Turkington TK, Friesen TL, Aboukhaddour R (2020) Parastagonospora nodorum and related species in Western Canada: genetic variability and effector genes. Phytopath 110:1946–1958
Halder J, Zhang J, Ali S, Sidhu JS, Gill HS, Talukder SK, Kleinjan J, Turnipseed B, Sehgal SK (2019) Mining and genomic characterization of resistance to tan spot, Stagonospora nodorum blotch (SNB), and Fusarium head blotch in Watkins core collection of wheat landraces. BMC Plant Biol 19:480
Hu W, He X, Dreisigacker S, Sansaloni CP, Juliana P, Singh PK (2019) A wheat chromosome 5AL region confers seedling resistance to both tan spot and Septoria nodorum blotch in two mapping populations. Crop J 7:809–818
International Wheat Yield Partnership (2017) Strategic Plan 2017–2022. http://iwyp.org/wp-content/uploads/sites/34/2017/10/IWYP-Strategic-Plan-Full-Version-Published.pdf, Accessed 4 April 2018
Jones JDG, Dangl JL (2006) The plant immune system. Nature 444:323–329
Juliana P, Singh RP, Singh PK, Crossa J, Rutkoski JE, Poland JA, Bergstrom GC, Sorrells ME (2017) Comparison of models and whole-genome profiling approaches for genomic-enabled prediction of Septoria tritici blotch, Stagonospora nodorum blotch, and Tan spot resistance in wheat. Plant Genome-US. https://doi.org/10.3835/plantgenome2016.08.0082
Kariyawasam GK, Richards JK, Wyatt NA, Running K, Xu SS, Liu Z, Borowicz P, Faris JD, Friesen TL (2021) The Parastagonospora nodorum necrotrophic effector SnTox5 targets the wheat gene Snn5 and facilitates entry into the leaf mesophyll. New Phytol. https://doi.org/10.1111/nph.17602
Kourelis J, van der Hoom RAL (2018) Defended to the nines: 25 years of resistance gene cloning identifies nine mechanisms for R protein function. Plant Cell 30:285–299
Lin M, Corsi B, Ficke A, Tan KC, Cockram J, Lillemo M (2020) Genetic mapping using a wheat multi-founder population reveals a locus on chromosome 2A controlling resistance to both lead and glume blotch caused by the necrotrophic fungal pathogen Parastagonospora nodorum. Theor Appl Genet 133:785–808
Lin M, Stadlmeier M, Mohler V, Tan KC, Ficke A, Cockram J, Lillemo M (2021) Identification and cross-validation of genetic loci conferring resistance to Septoria nodorum blotch using a German multi-founder winter wheat population. Theor Appl Genet 134:125–142
Lin SY, Chooi YH, Solomon PS (2018) The global regulator of pathogenesis PnCon7 positively regulates Tox3 effector gene expression through direct interaction in the wheat pathogen Parastagonospora nodorum. Mol Microbiol 109:78–90
Liu Z, El-Basyoni I, Kariyawasam G, Zhang G, Fritz A, Hansen J, Marais F, Friskop A, Chao S, Akhunov E, Baenziger PS (2015) Evaluation and association mapping of resistance to tan spot and stagonospora nodorum blotch in adapted winter wheat germplasm. Plant Dis 99:1333–1341
Liu ZH, Faris JD, Meinhardt SW, Ali S, Rasmussen JB, Friesen TL (2004a) Genetic and physical mapping of a gene conditioning sensitivity in wheat to a partially purified host-selective toxin produced by Stagonospora nodorum. Phytopathology 94:1056–1060
Liu Z, Faris JD, Oliver RP, Tan KC, Solomon PS, McDonald MC, McDonald BA, Nunez A, Lu S, Rasmussen JB, Friesen TL (2009) SnTox3 acts in effector triggered susceptibility to induce disease on wheat carrying the Snn3 gene. PLoS Pathog 5:e1000581
Liu Z, Friesen TL, Ling H, Meinhardt SW, Oliver RP, Rasmussen JB, Faris JD (2006) The Tsn1-ToxA interaction in the wheat-Stagonospora nodorum pathosystem parallels that of the wheat-tan spot system. Genome 49:1265–1273
Liu ZH, Friesen TL, Rasmussen JB, Ali S, Meinhardt SW, Faris JD (2004b) Quantitative trait loci analysis and mapping of seedling resistance to Stagonospora nodorum leaf blotch in wheat. Phytopathology 94:1061–1067
Liu Z, Gao Y, Kim YM, Faris JD, Shelver WL, de Wit PJGM, Xu SS, Friesen TL (2016) SnTox1, a Parastagonospora nodorum necrotrophic effector, is a dual-function protein that facilitates infection while protecting from wheat-produced chitinases. New Phytol 211:1052–1064
Liu Z, Zhang Z, Faris JD, Oliver RP, Syme R, McDonald MC, McDonald BA, Solomon PS, Lu S, Shelver WL, Xu S, Friesen TL (2012) The cysteine rich necrotrophic effector SnTox1 produced by Stagonospora nodorum triggers susceptibility of wheat lines harboring Snn1. PLoS Pathog 8:e1002467
Lozada D, Mason RE, Sarinelli JM, Brown-Guedira G (2019) Accuracy of genomic selection for grain yield and agronomic traits in soft red winter wheat. B C Genet 20:82
Lu H, Faris JD (2006) Macro- and microcolinearity between the genomic region of wheat chromosome 5B containing the Tsn1 gene and the rice genome. Funct Integr Gneomic 6:90–103
Lu HJ, Fellers JP, Friesen TL, Meinhardt SW, Faris JD (2006) Genomic analysis and marker development for the Tsn1 locus in wheat using bin-mapped ESTs and flanking BAC contigs. Theor Appl Genet 112:1132–1142
Lu Q, Lillemo M (2014) Molecular mapping of adult plant resistance to Parastagonospora nodorum leaf blotch in bread wheat lines ‘Shanghai-3/Catbird’ and ‘Naxos.’ Theor Appl Genet 127:2635–2644
Lu S, Faris JD, Sherwood R, Friesen TL, Edwards MC (2014) A dimeric PR-1-type pathogenesis-related protein interacts with ToxA and potentially mediates ToxA-induced necrosis in sensitive wheat. Mol Plant Pathol 15:650–663
Manning VA, Andrie RM, Trippe AF, Ciuffetti LM (2004) Ptr ToxA requires multiple motifs for complete activity. Mol Plant Microbe In 17:491–501
Manning VA, Ciufetti LM (2005) Localization of Ptr ToxA produced by Pyrenophora tritici-repentis reveals protein import into wheat mesophyll cells. Plant Cell 17:3203–3212
Manning VA, Chu AL, Steeves JE, Wolpert TJ, Ciufetti LM (2009) A host-selective toxin of Pyrenophora tritici-repentis, Ptr ToxA, induces photosystem changes and reactive oxygen species accumulation in sensitive wheat. Mol Plant Microbe In 22:665–676
Manning VA, Hardison LK, Ciufetti LM (2007) Ptr ToxA interacts with a chloroplast-localized protein. Mol Plant Microbe In 20:168–177
McDonald MC, Ahren D, Simpfendorfer S, Milgate A, Solomon PS (2017) The discovery of the virulence gene ToxA in the wheat and barley pathogen Bipolaris sorokiniana. Mol Plant Pathol 19:432–439
McDonald MC, Oliver RP, Friesen TL, Brunner PC, McDonald BA (2013) Global diversity and distribution of three necrotrophic effectors in Phaeosphaeria nodorum and related species. New Phytol 199:241–251
Meinhardt SW, Cheng W, Kwon CY, Donohue CM, Rasmussen JB (2002) Role of the arginyl-glycyl-aspartic motif in the action of the Ptr ToxA produced by Pyrenophora tritici-repentis. Plant Physiol 130:1545–1551
Navathe S, Yadav PS, Chand R, Mishra VK, Vasistha NK, Meher PK, Joshi AK, Gupta PK (2020) ToxA-Tsn1 interaction for spot blotch susceptibility in Indian wheat: an example of inverse gene-for-gene relationship. Plant Dis 104:71
Oliver RP, Friesen TL, Faris JD, Solomon PS (2012) Stagonospora nodorum: from pathology to genomics and host resistance. Ann Rev Phytopathol 50:23–43
Oliver RP, Lord M, Rybak K, Faris JD, Solomon PS (2008) Emergence of Tan Spot disease caused by toxigenic Pyrenophora tritici-repentis in Australia is not associated with increased deployment of toxin-sensitive cultures. Phytopathol. https://doi.org/10.1094/PHYTO-98-5-0488
Pandelova I, Betts MF, Manning VA, Wilhelm LJ, Mockler TC, Ciuffetti LM (2009) Analysis of transcriptome changes induced by Ptr ToxA in wheat provides insights into the mechanisms of plant susceptibility. Mol Plant 2:1067–1083
Peters Haugrud AR, Zhang Z, Richards JK, Friesen TL, Faris JD (2019) Genetics of variable disease expression conferred by inverse gene-for-gene interactions in the wheat-Parastagonospora nodorum pathosystem. Plant Physiol 180:420–434
Phan HTT, Furuki E, Hunziker L, Rybak K, Tan KC (2021) GWAS analysis reveals distinct pathogenicity profiles of Australian Parastagonospora nodorum isolates and identification of marker-trait-associations to Septoria nodorum blotch. Sci Rep-UK 11:10085
Phan HTT, Rybak K, Bertazzoni S, Furuki E, Dinglasan E, Hickey LT, Oliver RP, Tan KC (2018) Novel sources of resistance to Septoria nodorum blotch in the Vavilov wheat collection identified by genome-wide association studies. Theor Appl Genet 131:1223–1238
Phan HTT, Rybak K, Furuki E, Breen S, Solomon PS, Oliver RP, Tan KC (2016) Differential effector gene expression underpins epistasis in a plant fungal disease. Plant J 87:343–354
Paillard P, Schurbusch T, Winzeler M, Messmer M, Sourdille P, Abderhalden O, Keller B, Schachermayr G (2003) An integrative genetic linkage map of winter wheat (Triticum aestivum L.). Theor Appl Genet 115:313–323
Reddy L, Friesen TL, Meinhardt SW, Chao S, Faris JD (2008) Genomic analysis of the Snn1 locus on wheat chromosome arm 1BS and the identification of candidate genes. Plant Genome 1:55–66
Richards JK, Kariyawasam G, Seneviratne S, Wyatt NA, Xu SS, Liu Z, Faris JD, Friesen TL (2021) A triple threat: the Parastagonospora nodorum SnTox267 effector exploits three distinct host genetic factors to cause disease in wheat. New Phytol. https://doi.org/10.1111/nph.17601
Ruud AK, Dieseth JA, Ficke A, Furuki E, Phan HTT, Oliver RP, Tan KC, Lillemo M (2019) Genome-wide association mapping of resistance to Septoria nodorum leaf blotch in a Nordic spring wheat collection. Plant Genome 12:180105
Ruud AK, Dieseth JA, Lillemo M (2018) Effects of three Parastagonospora nodorum necrotrophic effectors on spring wheat under Norwegian field conditions. Crop Sci 58:159–168
Ruud AK, Windju S, Belova T, Friesen TL, Lillemo M (2017) Mapping of SnTox3-Snn3 as a major determinant of field susceptibility to Septoria nodorum blotch in the SHA3/CBRD × Navos population. Theor Appl Genet 130:1361–1374
Rybak K, See PT, Phan HTT, Syme RA, Moffat CS, Oliver RP, Tan KC (2017) A functionally conserved Zn2Cys6 binuclear cluster transcription factor class regulates necrotrophic effector gene expression and host-specific virulence of two major Pleopsporales fungal pathogens of wheat. Mol Plant Pathol 18:420–434
Ryerson DE, Heath MC (1996) Cleavage of nuclear DNA into oligonucleosomal fragments during cell death induced by fungal infection or by abiotic treatments. Plant Cell 8:393–402
Sarinelli JM, Murphy JP, Tyagi P, Holland JB, Johnson JW, Mergoum M, Mason RE, Babar A, Harrison S, Sutton R, Griffey CA, Brown-Guedira G (2019) Training population selection and use of fixed effects to optimize genomic predictions in a historical USA winter wheat panel. Theor Appl Genet 132:1247–1261
Shankar M, Walker E, Golzar H, Loughman R, Wilson RE, Francki MG (2008) Quantitative trait loci for seedling and adult plant resistance to Stagonospora nodorum in Wheat. Phytopathology 98:886–893
Sharma S (2019) Genetics of wheat domestication and Septoria nodorum blotch susceptibility in wheat. Masters thesis, North Dakota State University
Shatalina M, Messmer M, Feuillet C, Mascher F, Paux E, Choulet F, Wicker T, Keller B (2014) High-resolution analysis of a QTL for resistance to Stagonospora nodorum glume blotch in wheat reveals presence of two distinct resistance loci in the target interval. Theor Appl Genet 127:573–586
Shi G, Friesen TL, Saini J, Xu SS, Rasmussen JB, Faris JD (2015) The wheat Snn7 gene confers susceptibility on recognition of the Parastagonospora nodorum necrotrophic effector SnTox7. Plant Genome. https://doi.org/10.3835/plantgenome2015.02.0007
Shi G, Zhang Z, Friesen TL, Bansal U, Cloutier S, Wicker T, Rasmussen JB, Faris JD (2016a) Marker development, saturation mapping, and high-resolution mapping of the Septoria nodorum blotch susceptibility gene Snn3-B1 in wheat. Mole Genet Genom 291:107–119
Shi G, Zhang Z, Friesen TL, Raats D, Fahima T, Brueggeman RS, Lu S, Trick HN, Liu Z, Chao W, Frenkel Z, Xu SS, Rasmussen JB, Faris JD (2016) The hijacking of a receptor kinase-driven pathway by a wheat fungal pathogen leads to disease. Sci Adv 2:e1600822
Singh M, Upadhyaya HD (2015) Genetic and genomic resources for grain cereals improvement. Academic Press, Cambridge
Singh PK, Singh S, Deng Z, He X, Kehel Z, Singh RP (2019) Characterization of QTLs for seedling resistance to Tan spot and Septoria nodorum blotch in the PBW343/Kenya Nyangumi wheat recombinant inbred lines population. Int J Mol Sci 20:5432
Singh PK, Singh RP, Duveiller E, Mergoum M, Adhiikari TB, Elias EM (2010) Genetics of wheat-Pyrenophora tritici-repentis interactions. Euphytica 171:1–13
Singh RP, Singh PK, Rutkoski J, Hodson DP, He X, Jørgensen LN, Hovmøller MS, Huerta-Espino J (2016) Disease impact on wheat yield potential and prospects of genetic control. Ann Rev Phytopathol 54:303–322
Sung YC, Outram MA, Breen S, Wang C, Dagvadorj B, Winterberg B, Kobe B, Williams SJ, Solomon PS (2020) PR1-mediated defence via C-terminal peptide release is targeted by a fungal pathogen effector. New Phytol 229:3467–3480
Tai YS, Bragg J, Meinhardt S (2007) Functional characterization of ToxA and molecular identification of its targeting protein in wheat. Am J Plant Physiol 2:76–89
Tan KC, Waters ODC, Rybak K, Antoni E, Furuki E, Oliver RP (2014) Sensitivity to three Parastagonospora nodorum necrotrophic effects in current Australian wheat cultivars and the presence of further fungal effectors. Crop Pasture Sci 65:150–158
Tomás A, Bockus WW (1987) Cultivar specific toxicity of culture filtrate of Pyrenophora tritici-repentis. Phytopathology 77:1337–1366
Tuori RP, Wolpert TJ, Ciuffetti LM (1995) Purification and immunological characterization of toxic components from cultures of Pyrenophora tritici-repentis. Mol Plant-Microbe Interact 8:41–48
Tuori RP, Wolpert TJ, Ciuffetti LM (2000) Heterologous expression of functional Ptr ToxA. Mol Plant-Microbe Interact 13:456–464
van Schie CCN, Takken FLW (2014) Susceptibility genes 101: how to be a good host. Ann Rev Phytopathol 52:551–581
Vincent D, Du Fall LA, Livk A, Mathesius U, Lipscombe RJ, Oliver RP, Friesen TL, Solomon PS (2012) A functional genomics approach to dissect the mode of action of the Stagonospora nodorum effector protein SnToxA in wheat. Mol Plant Pathol 13:467–482
Virdi SK, Liu Z, Overlander ME, Zhang Z, Xu SS, Friesen TL, Faris JD (2016) New insights into the roles of host gene-necrotrophic effector interactions in governing susceptibility of durum wheat to tan spot and spetoria nodorum blotch. G3-Genes Genom Genet 6:4139–4150
Waters ODC, Lichtenzveig J, Rybak K, Friesen TL, Oliver RP (2011) Prevalence and importance of sensitivity to the Stagonospora nodorum necrotrophic effector SnTox3 in current Western Australian wheat cultivars. Crop Pasture Sci 62:556–562
Winterberg B, Du Fall LA, Song X, Pascovici D, Care N, Molloy M, Ohms S, Solomon PS (2014) The necrotrophic effector protein SnTox3 re-programs metabolism and elicits a strong defence response in susceptible wheat leaves. BMC Plant Biol 14:215
Zhang H, Francl LJ, Jordahl JG, Meinhardt SW (1997) Structural and physical properties of a necrosis-inducing toxin from Pyrenophora tritici-repentis. Phytopathology 87:154–160
Zhang Z, Friesen TL, Simons KJ, Xu SS, Faris JD (2009) Development, identification, and validation of markers for marker-assisted selection against the Stagonospora nodorum toxin sensitivity genes Tsn1 and Snn2 in wheat. Mol Breed 23:35–49
Zhang Z, Friesen TL, Xu SS, Shi G, Liu Z, Rasmussen JB, Faris JD (2011) Two putatively homoeologous wheat genes mediate recognition of SnTox3 to confer effector-triggered susceptibility to Stagnonospora nodorum. Plant J 65:27–38
Zhang Z, Running KLD, Seneviratne S, Peters Haugrud AR, Szabo-Hever A, Shi G, Brueggeman R, Xu SS, Friesen TL, Faris JD (2021) A protein kinase-major sperm protein gene hijacked by a necrotrophic fungal pathogen triggers disease susceptibility in wheat. Plant J. https://doi.org/10.1111/tpj.15194
Zhang W, Zhu X, Zhang M, Shi G, Liu Z, Cai X (2019) Chromosome engineering-mediated introgression and molecular mapping of novel Aegilops speltoides-derived resistance genes for tan spot and Septoria nodorum blotch diseases in wheat. Theor Appl Genet 132:2605–2614
Funding
This funding was provided by Agricultural Research Service, 3060–21000-038-00D, Justin Faris.
Author information
Authors and Affiliations
Contributions
ARPH and JDF wrote and edited original versions of the manuscript. ZZ and TLF participated in editing and modifying the final version.
Corresponding author
Additional information
Communicated by Hermann Buerstmayr.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Peters Haugrud, A.R., Zhang, Z., Friesen, T.L. et al. Genetics of resistance to septoria nodorum blotch in wheat. Theor Appl Genet 135, 3685–3707 (2022). https://doi.org/10.1007/s00122-022-04036-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-022-04036-9